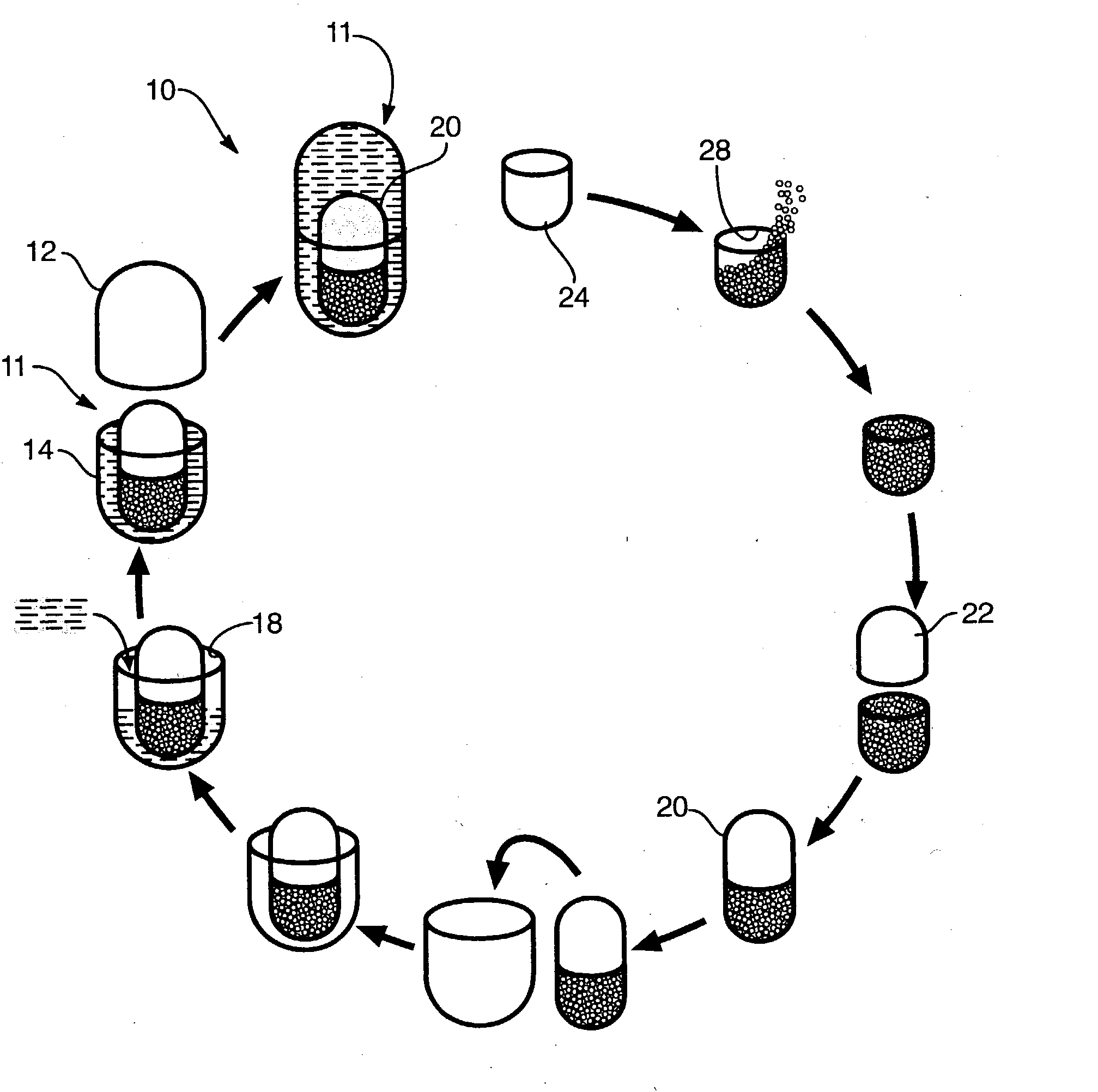
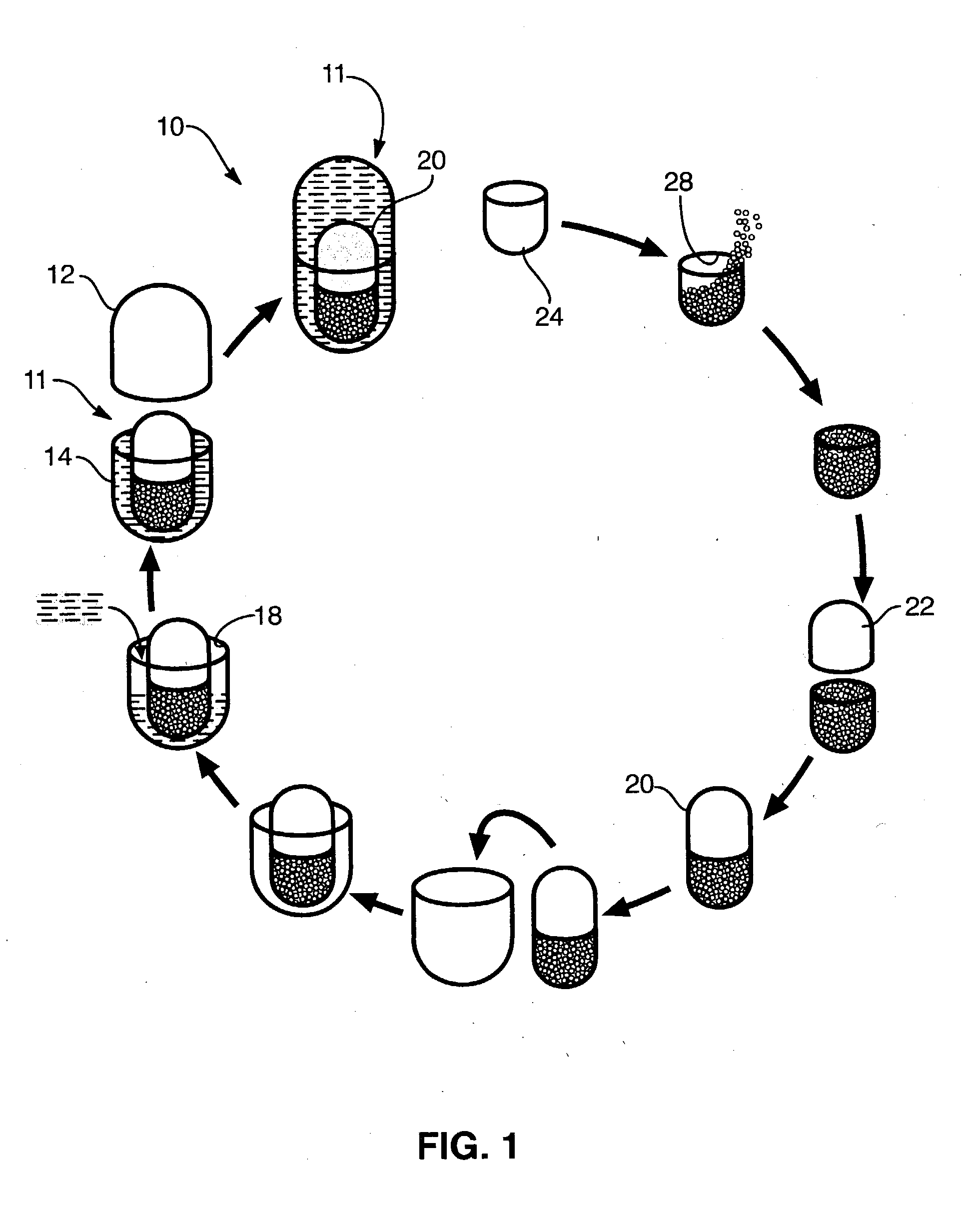
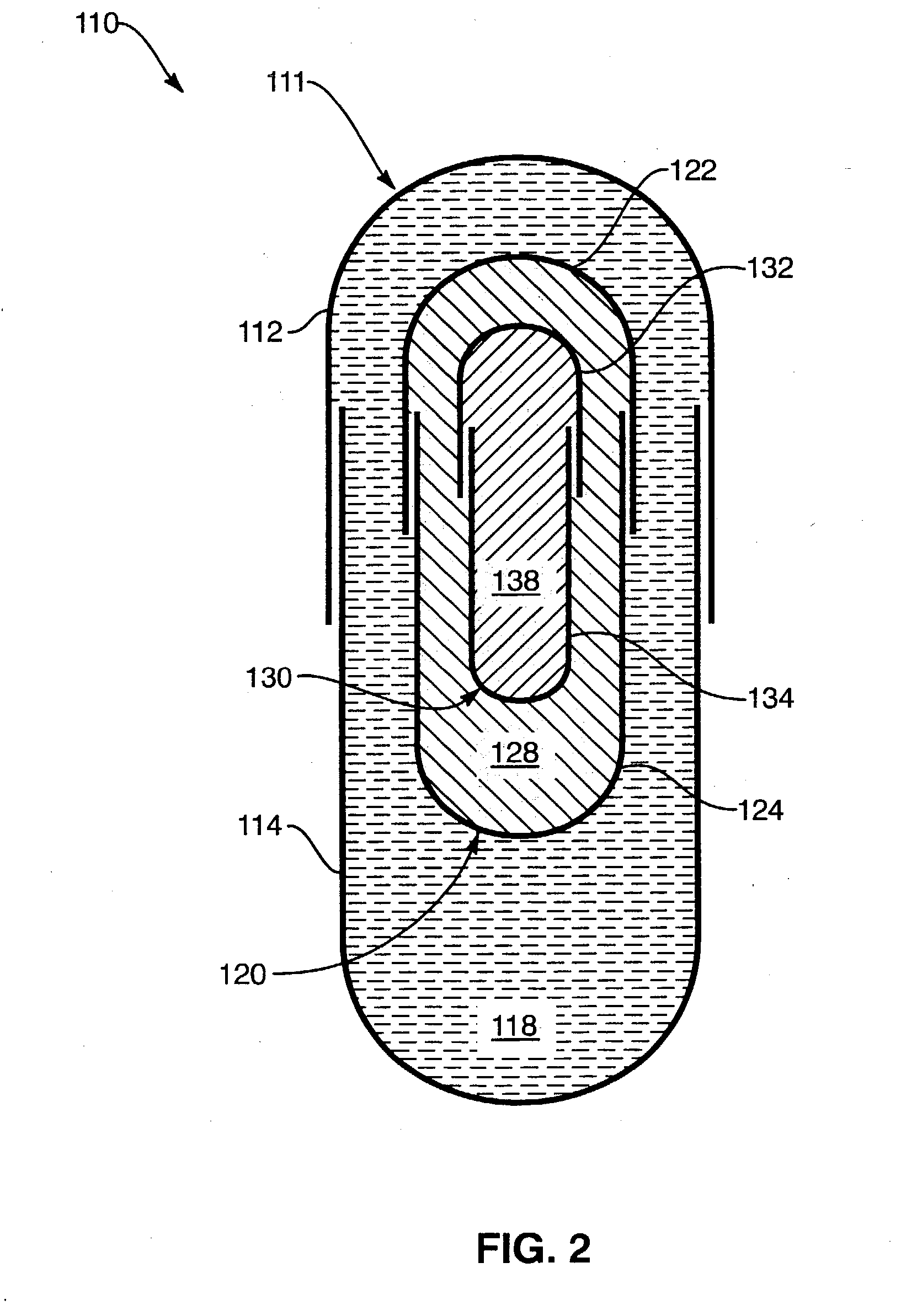
Process for encapsulating multi-phase, multi-compartment capsules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
[Glucosamine / Chondroitin (Solid) & Vitamin E (Liquid)]
[0109] As appreciated by those skilled in the art, arthritis is an inflammatory condition typically affecting the synovia membranes and cartilage of joints. It has been estimated that as many as one in three persons may experience symptoms associated with arthritis during their lifetime.
[0110] In addition to arthritis, various other chronic, debilitating conditions may afflict the aged. Many of these conditions result from the natural process of aging in humans. The natural aging process is partially due to the accumulation and effects of toxic free-radical chemicals. Free-radicals result from several homeostatic biochemical processes. It is, accordingly, desirable to develop nutraceutical or dietary supplement products which may alleviate multiple chronic, debilitating conditions. It is also desirable to package and administer such products in the most economic and convenient possible fashion.
[0111] The administration of glucosa...
example ii
[S-adenosylmethione (SAMe) (Solid) & Vitamin E (Liquid)]
[0117] S-adenosylmethione (SAMe), may be derived from two materials: methionine, a sulfur-containing amino acid, and adenosine triphosphate (ATP), the body's main energy compound. SAMe was originally developed around 1950 as an antidepressant. Over the years, it has also been found that SAMe may assist in alleviating arthritic symptoms, assist in the manufacture of melatonin, which is needed to regulate sleep, help protect DNA from harmful mutations and prevent certain types of nerve damage.
[0118] As noted above, vitamin E is a popular anti-oxidant, but it is poorly soluble in water and therefore can be administered only as a liquid-oil formulation. Vitamin E is typically measured in international units (IU) of alpha tocopherol.
[0119] In one presently preferred embodiment of the present invention, therapeutically effective amounts of SAMe and vitamin E (active ingredients) may be introduced into receiving chambers of a multi-co...
example iii
[Curcumin, Holy Basil, Zinc (Solid) & Fish Oil (Omega 3 Fatty Acids DHA & EPA--Liquid)]
[0124] Curcumin belongs to a class of compounds derived from the turmeric root and is a yellow-orange volatile oil. It is believed that curcumin has an inhibitory effect on carcinogenesis, which is the evolution of a normal cell into a cancerous cell. There is clinical evidence to suggest curcumin may help to prevent stomach, colon, oral, esophageal, breast and skin cancers. Additional studies have been conducted to show that curcumin may be helpful in balancing cholesterol levels, protecting against ulcers by inhibition of gastric acid secretion and protection of gastric mucosal tissue, and anti-inflammatory actions. In one clinical study, curcumin was found to be as effective as non-steroidal anti-inflammatory drugs in the treatment of arthritis and post-operative pain.
[0125] The administration of Holy Basil (Ocimum sanctum) has been shown to have an effect on promoting peripherally mediated ana...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com