Separating agent for optical isomer
a technology of optical isomers and separation agents, which is applied in the separation of optically active compounds, chemical/physical processes, and esterified saccharide compounds, etc., can solve the problems of unsuitable maintenance of analytical equipment, complicated replacement between organic solvent-based mobile phases and aqueous mobile phases, and significant differences in medical effects and toxicities between the two enantiomeric isomers. , to achieve the effect of minimizing uv absorption and high asymmetry recognition ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0030] Production of Cellulose
[0031] Tris(cyclohexylcarboxylate)-Supported Filler and Packed Column
[0032] (1) Synthesis of Cellulose Tris(cyclohexylcarboxylate) (a) To 15 ml of N,N-dimethylacetamide (DMAc), 1.5 g of vacuum-dried lithium chloride was dissolved to prepare a DMAc / LiCl solution.
[0033] Under a nitrogen atmosphere, 15 ml of the above DMAc / LiCl solution and 15 ml of pyridine were added to 1.0 g of cellulose, and the resulting mixture was dipped in an oil bath at 100.degree. C. and stirred for 24 hrs. Thereafter, 4.8 g of chloride cyclohexylcarboxylate (C.sub.6H.sub.11COCl) (34 mmol, 1.8 equivalents) was added thereto and reacted for 16 hrs at 100.degree. C. The resulting solution was dripped in 200 ml of methanol and reprecipitated followed by centrifugal precipitation. As a result, the intended ester derivative (2.8 g, 93%) was obtained. The elemental analytic result of the resulting ester derivative (a) is shown in Table 1.
1TABLE 1 Elemental Analytic Result of Cellulose ...
applied example 1
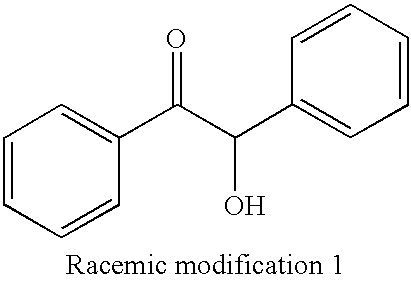
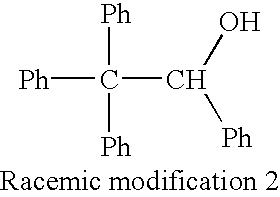
[0052] The enantiomeric isomer separation columns obtained in Example 1 and Comparative Examples 1-2 were used to perform the evaluation of asymmetry recognition ability for racemic modifications 1 and 2 represented by the following formulae by a liquid chromatography of the following condition. The result is shown in Table 2.
[0053]
2 Example 1: H.sub.2O / MeOH = 2.8 (v / v) Mobile Phase Comparative Examples 1 and 2: MeOH Flow Velocity 0.5 ml / min Temperature 25.degree. C.
[0054]
3 TABLE 2 1 2 Separating Agent Separation Factor (.alpha.) Comparative Comparative Racemic Modification Example 1 Example 1 Example 2 Racemic Modification 1 1.64 1.0 1.37 Racemic Modification 2 1.16 1.0 1.0
example 2
[0055] Production of Cellulose Tris(cyclopentylcarboxylate)-Supported Filler and Packed Column
[0056] (1) Synthesis of Cellulose Tris(cyclopentylcarboxylate) (d)
[0057] To 15 ml of N,N-dimethylacetamide (DMAc), 1.5 g of vacuum-dried lithium chloride was dissolved to prepare a DMAc / LiCl solution.
[0058] Under a nitrogen atmosphere, 15 ml of the DMAc / LiCl solution and 15 ml of pyridine were added to 1.0 g of cellulose, and the mixture was dipped in a hot water bath at 100.degree. C. and stirred for 24 hrs. Thereafter, 4.4 g of chloride cyclopentylcarboxylate (33 mmol, 1.8 equivalent) was added thereto and reacted at 100.degree. C. for 16 hrs. The reaction dope was poured into 200 ml of MeOH and reprecipitated followed by centrifugal separation. As a result, the intended ester derivative was obtained (2.5 g, 90%). The elementary analytic result of (d) is shown in Table 3.
[0059] (2) Production of Filler With Cellulose Tris(cyclopentylcarboxylate-) Supported Thereon
[0060] To 10 ml of tetrah...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com