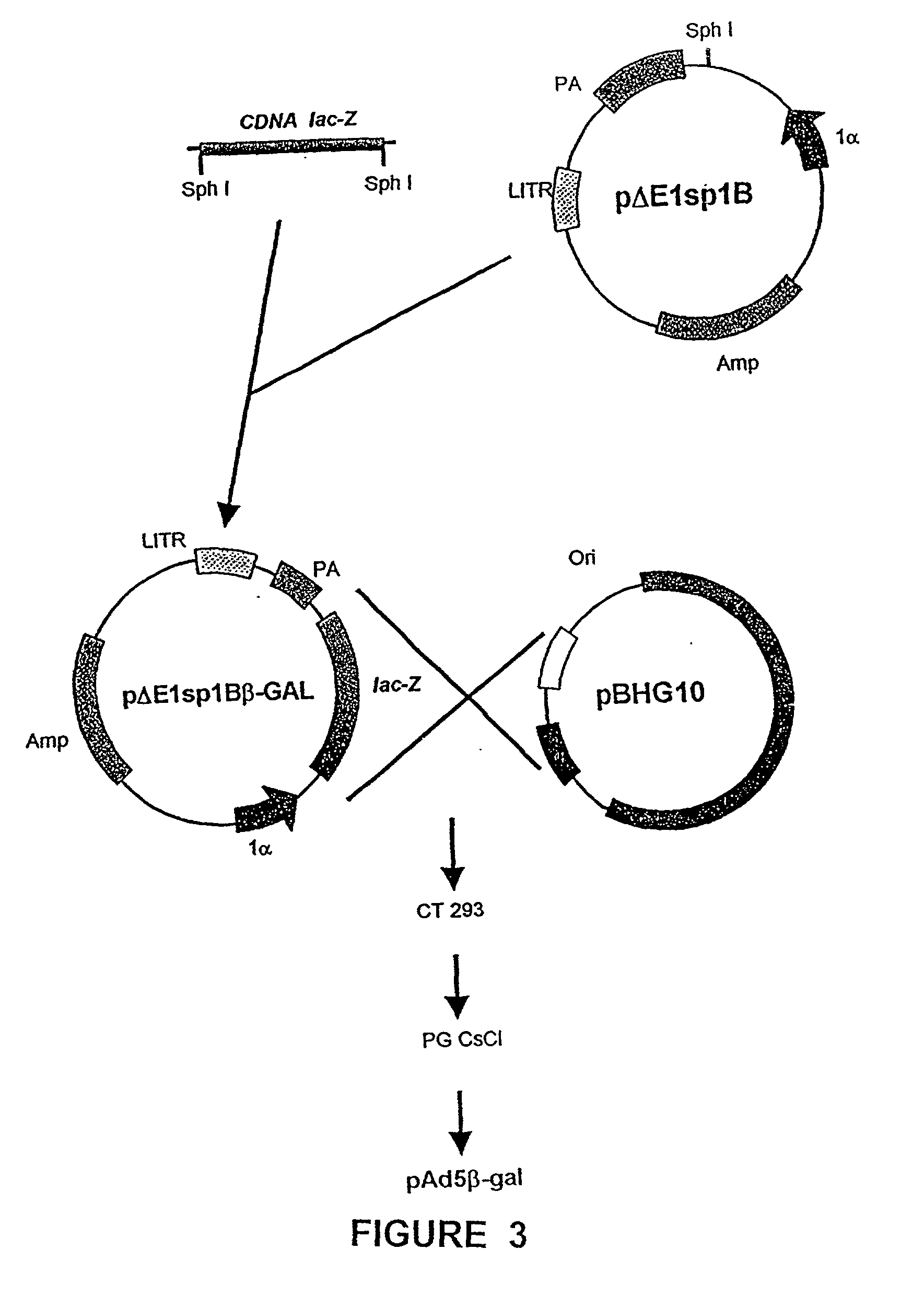
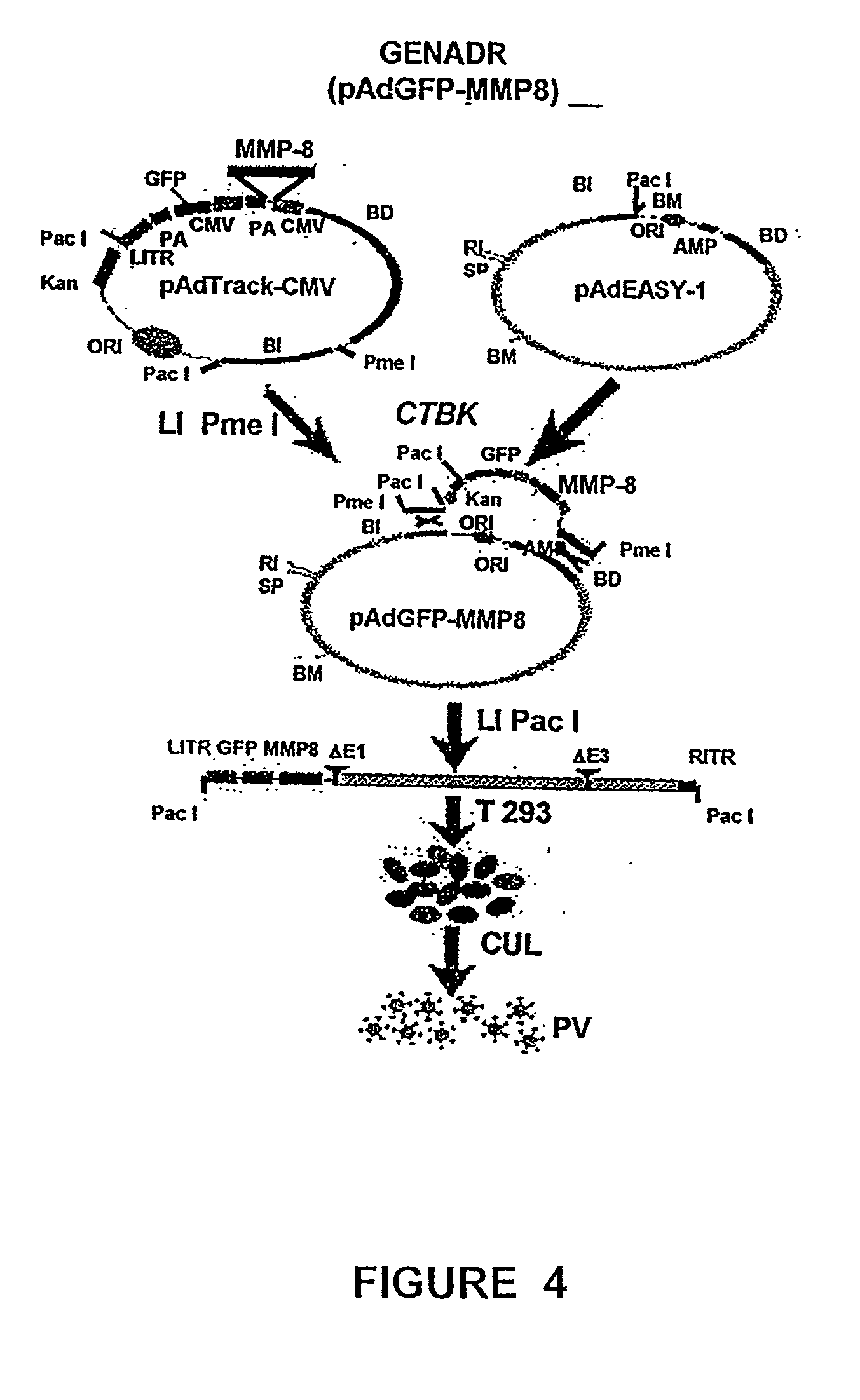
Recombinant adenoviral vectors and their utility in the treatment of various types of fibrosis: hepatic, renal, pulmonary, as well as hypertrophic scars
a technology of adenoviral vectors and fibrosis, which is applied in the direction of plant growth regulators, enzymes, biocide, etc., can solve the problems of patient's death, liver not being able to maintain the physiologic concentration of solutes, and hepatic failur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0073] Methodology to demonstrate the activity of Metalloprotease or Collagenase (MMP-8) and how to regulate its function
[0074] a) Cell Culture
[0075] HepG2 cells is a cell line of parenchymal origin derived from a human hepatoma, and were cultured in 60 mm culture dish, 37.degree. C. in a wet atmosphere, with 95% air and CO.sub.2 5% atmosfere in Eagle's medium modified by Dulbecco (DMEM), supplemented with 10% fetal bovine serum, 2 mM L-Glutamax and antibiotics (100 U / ml penicillin and 100 g. / ml. streptomycin).
[0076] b) Vectors of Expression of Latent and Active MMP-8 Genes
[0077] Two plasmids were used with 2 kinds of MMP-8 genes to transfect the hepatic cells: The plasmid pcDNA-MMP-8 which contains the cDNA which encodes for latent MMP-8 (pro-MMP-8) together with the strong viral promoter of cytomegalovirus (CMV); and the plasmid pcDNA.sub.3MMP-8 containing the cDNA which encodes for the active MMP-8, together with the CMV promoter. This last one was created through subclonation us...
example 2
[0098] Results to Demostrate the Activity of Metalloprotease or Collagenase (MMP-8) and therefore to Regulate its Function
[0099] Subcloning permitted to incorporate MMP-8 cDNA encoding for the fully functional enzyme was subcloned in a vector appropriate to our needs. Thus, FIG. 15 shows an electrophoresis of the DNA fragments released by cutting MMP-8 plasmids with restriction enzymes. Lane A). Marker of bp of 1 Kb DNA ladder (Gibco BRL); B). Perfect DNA marker (Novagen, Inc.); 1) pcDNA-MMP-8 cutting with BamHI and XbaI; 2) pcDNA3-MMP-8 cutting with BamHI and XbaI; C) .phi.X174 marker (Gibco BRL); .lambda. Hind III Marker (Gibco BRL), in which the latent MMP-8 cDNA (lane 1) and the mature MMP (lane 2); were successfully subcloned in the expression vectors pcDNA and pcDNA3. The released inserts are observed after treatment with restriction enzymes BamH1 and XbaI. The bands stained with ethydium bromide correspond to each of cDNA (between 506 and 560 base pairs) for mature and latent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com