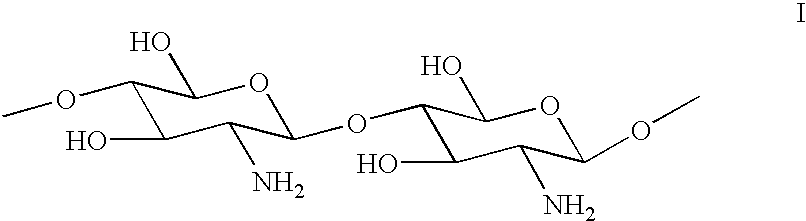
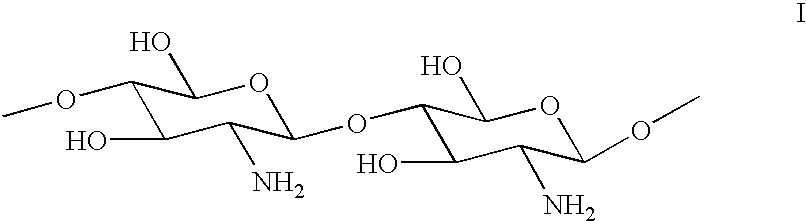
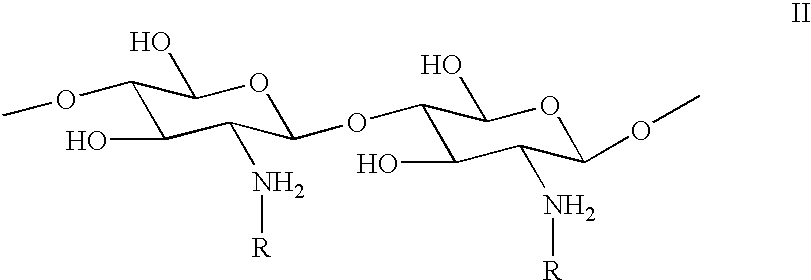
Method for the treatment of textiles after washing
a textile and textile technology, applied in the field of textile treatment, can solve the problems of residual detergents, no effective solution to residual detergent problems, and increase in the amount of antifoaming agents in the formulas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experiment example 2
[0059] FIG. 4 shows the changes of pH value after 5% chitosan solution is added respectively in 2L water dissolving 0.1 g of A detergent, 2L water dissolving 0.2 g of A detergent, and 2L water. This experiment is conducted by stirring 5 min and then testing the pH value to simulate the case of chitosan adjustment after the base of the detergent remains. The result shows that even if a little amount of chitosan is added, it is effective in neutralizing the basicity of water and lowering the pH value.
experiment example 3
[0060] FIGS. 5a and 5b show the reduction of surfactant activities of the anionic surfactant due to chitosan. In FIG. 5a, 5 g of A detergent is dissolved in 12 L water. 1 g of 9% C cationic surfactant is added directly into 150g of the above detergent solution. After standing 20 min, the precipitants happen. C cationic surfactant is combined with anionic surfactants in water to form cloudy precipitants. In FIG. 5b, 4 g chitosan solution containing 5% chitosan and 2% acetic acid is first added into 150 g of same detergent solution, and then 1 g of 9% C cationic surfactant is added. After standing 20 min, it is found that the addition of chitosan does effectively reduce the surfactant activities of the anionic surfactant and thus reduce the combination with the cationic surfactant. From the result of experiments, the more chitosan added, the better the effect of reducing the surfactant activities of the anionic surfactant and the less precipitant happen.
experiment example 4
[0061] When 50 g of commercial softener is neutralized with 0.1N NaOH, the changes of the pH values with or without addition of chitosan are shown in FIG. 6. From the experimental data of the FIG. 6, all the pH values of the commercial textile softeners A, B, C, and D are rapidly increased in basic environment, and the viscosity is thus increased. Since the major components of the softener all are positively charged cationic surfactants, the pH value may seriously affect the amount of charges and may further affect its function. From the figure, it also shows some brands prepare the softener with a lower pH value. Although an acidic environment is good for increasing the amount of charges in the cationic surfactants, it is not good for human skin. And it has no resistance to the environment, that is, it has no effect of practical adjusting environment. If excess acid is added without neutralization, it harms both the human body and the textile. After sample C is added with chitosan ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com