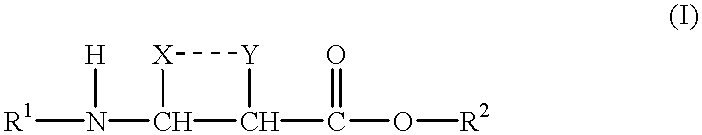
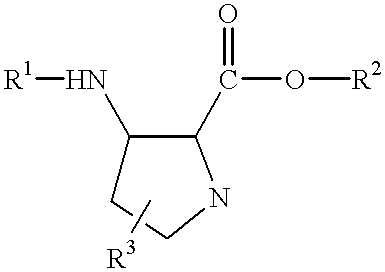
Beta-amino acids
a technology of amino acids and beta-amino acids, which is applied in the field of beta-amino acids, can solve the problems of uncontrolled aggregation of bulk peptides, contradictory prior art reports on the structure of beta-amino acids, and inability to synthesize reactions, etc., and achieve high conformational stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0136] Amide Proton Exchange in trans-ACHC Dimer and Hexamer:
[0137] Amide proton exchange is one of the most powerful methods for assessing conformational stability of peptides and proteins; adoption of a stable intramolecularly hydrogen-bonded conformation leads to diminution of the rate of exchange. NH / ND exchange behavior of the trans-ACHC hexamer relative to the corresponding dimer (which is too small to form a favorable internal hydrogen bond) shows that the hexamer adopts a very stable intramolecularly hydrogen-bonded folding pattern in methanol solution.
[0138] To ensure a direct comparison, this Example was conducted with solutions containing 2 mM of the trans-ACHC dimer and 2 mM of the trans-ACHC hexamer. The .sup.1H NMR results are shown in FIG. 3. Upon dissolution of the 1:1 dimer:hexamer mixture in CD.sub.3OD, the amide proton and the urethane proton of the dimer (marked with asterisks) are completely exchanged within 6 min, according to .sup.1H NMR (FIG. 3). In contrast,...
example 2
[0140] Circular Dichroism and NOE's in trans-ACPC
[0141] As noted above, computational comparisons involving alternative cycloalkane-based .beta.-amino acids and alternative P -peptide helices suggested that the trans-ACHC / 14-helix combination would generate a stable .beta.-peptide secondary structure. Using the same technique, the trans-ACPC / 12-helix combination was predicted to be almost as favorable. This latter prediction is intriguing because there is no precedent for the 12-helix in the contradictory literature on polymers constructed from optically active .beta.-amino acids. Among these polymers, poly(.alpha.-isobutyl-L-aspartate) has been particularly intensively studied, and proposed secondary structures include 14-, 16-, 18- and 20-helix, as well as sheet. Since the computational predictions regarding the trans-ACHC / 14-helix relationship described above proved to be correct, the trans-ACPC / 12-helix prediction was then explored.
[0142] Optically active trans-ACPC was prepared...
example 3
[0149] Amide Proton Exchange in Amino--Substituted trans-ACHA / trans ACHA Dimers and Hexamers:
[0150] In this Example, amino-substituted-trans-ACHA (i.e., ACHA containing an exocyclic amino substituent) was synthesized according to Reaction 3, above. The amino-substituted-trans-ACHA was then coupled with unsubstituted trans-ACHA as shown in Reaction 7 to yield .beta.-peptides wherein the residues alternate between unsubstituted-trans-ACHA and amino-substituted-trans-ACHA. These molecules were synthesized because it was anticipated that the amino group would be protonated in water and that the resulting positive charge would render these .beta.-peptides water-soluble. They are indeed water-soluble.
[0151] The amide exchange of the dimer and the hexamer were then compared in the same fashion as described in Example 1 in order to probe for 14-helix formation in water. FIG. 6 depicts the two-dimensional .sup.1H NMR data obtained for the alternating unsubstituted-trans-ACHA / amino-subs-titut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| air pressure | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com