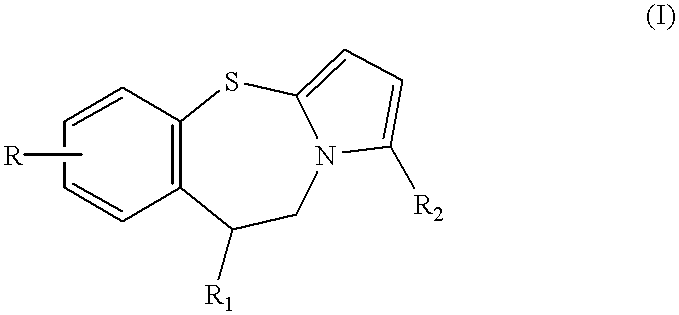
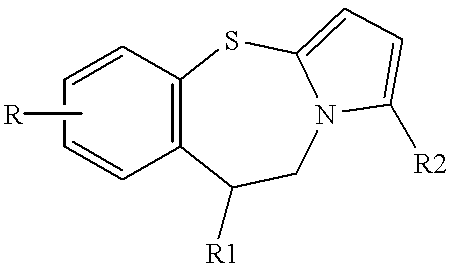
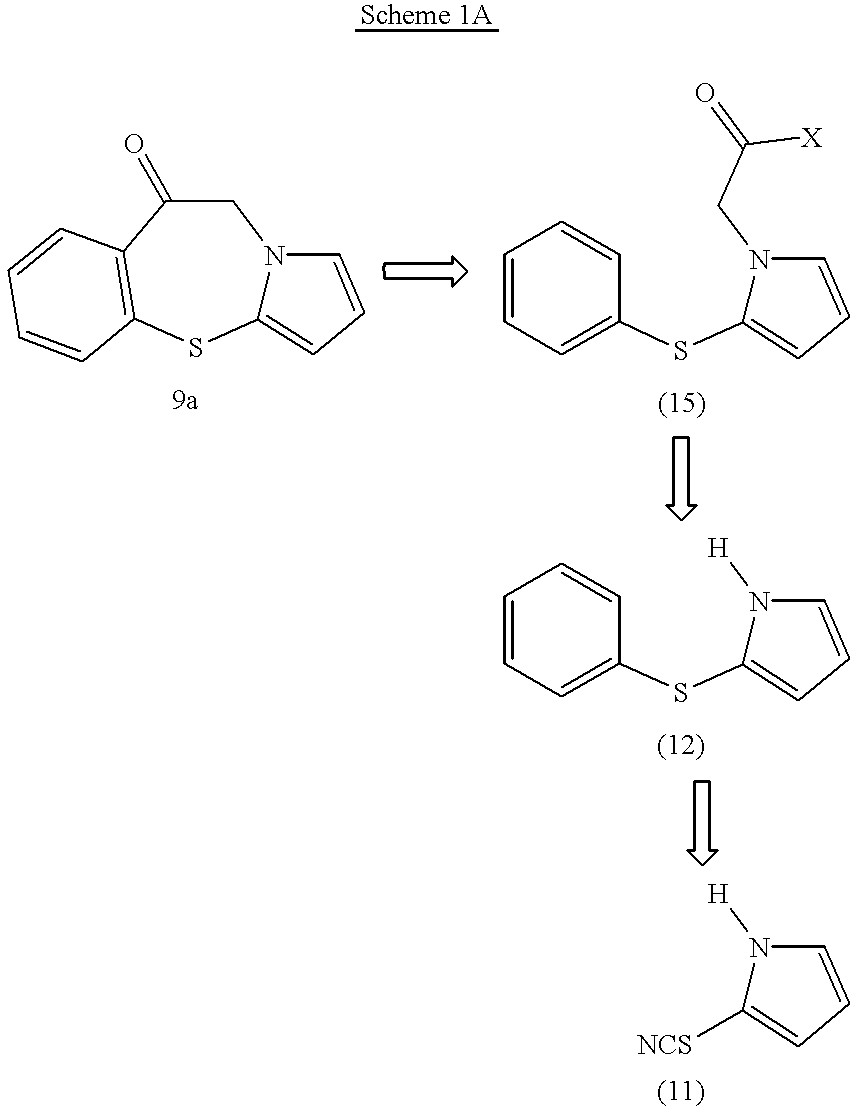
Pyrrolo {2,1-b}{1,3}benzothiazepines with atypical antipsychotic activity
a technology of benzothiazepines and pyrrolol, which is applied in the direction of biocide, organic chemistry, drug compositions, etc., can solve the problems of inferior general potency, unsatisfactory need for antipsychotic agents with substantial therapeutic activity, and limited therapeutic use, so as to reduce the dose needed, prolong the exposure to the drug, and reduce the effect of toxicity and accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0099] (.+-.)-9-(4-methylpiperazin-1-yl)-9,10-dihydropyrrolo[2,1-b][1,3]en-zo-thiazepine (3a)
[0100] A mixture of 25a (0.65 g, 2.0 mmol) and N-methylpiperazine (1.1 ml, 10.0 mmol) was heated to 130.degree. C. for 2 hours under argon, cooled, poured onto crushed ice and extracted with ethyl ether. The organic extracts collected were washed with brine, anhydrified. and concentrated. The residue was chromatographed (EtOAc) to give 0.45 g (75% yield) of 3a as colourless prisms: melting point 206-207.degree. C. (hexane); .sup.1H NMR (CDCl.sub.3) .delta.7.49-7.09 (m, 4H); 6.87 (m, 1H), 6.29 (m, 1H), 4.68 (dd, 1H, J=14.4, 8.6 Hz), 4.51 (dd 1H, J=14.4, 3.7 Hz), 2.56-2.34 (m, 8H), 2.23 (s, 3H); .sup.13C NMR (CDCl.sub.3) .delta.138.1, 134.6, 132.9, 130.4, 127.3, 126.9, 123.9, 121.7, 113.3, 107.7, 66.1, 55.9, 48.8, 46.6. 46.1. MS m / z 299 (100, M.sup.+), 219, 200, 167, 149, 113. Anal. (C.sub.17H.sub.21N.sub.3S) C, H, N.
example 2
[0101] (.+-.)-7-chloro9-(4-methylpiperazin-1-yl)-9,10-dihydropyrrolo[2,1-b-][1,3]benzothiazepine (3b) (ST1455)
[0102] The titre compound was obtained starting from 25b (0.3 g, 0.95 mmol) using the procedure described above. 3b was obtained as colourless prisms (68% yield): melting point 210-211.degree. C. (hexane); .sup.1H NMR (CDCl.sub.3) .delta.7.51 (d, 1H, J=2.4 Hz); 7.30 (d, 1H, J=8.5 Hz), 7.06 (dd, 1H, J=8.5, 2.4 Hz), 6.86 (m, 1H), 6.29 (m, 1H), 6.05 (m, 1H), 4.71 (dd, 1H, J=14.0, 8.6 Hz), 4.45 (dd, 1H, J=14.0, 3.4 Hz), 3.95 (dd, 1H, J=8.6, 3.4 Hz), 2.65-2.25 (m, 8H), 1.42 (s, 3H); .sup.13C NMR (CDCl.sub.3) .delta.140.1, 133.2, 133.0, 132.4, 131.6, 127.3, 123.9, 121.1, 113.6, 107.9, 65.9, 55.8, 55.7, 47.7, 45.9, 44.9, 26.8. MS m / z 333 (10, M.sup.+), 250, 233 (100), 201, 166, 139. Anal. (C.sub.17H.sub.20CIN.sub.3S): C, H, N. The dihydrochloride salt (named hereinafter ST1468) was obtained by dissolving an analytical sample in HCl 1N in methanol. The solvent was evaporated and the...
example 3
[0103] Semipreparatory Chiral Separation of (.+-.)-3b
[0104] First of all, the hydrochloride salt of (.+-.)-3b was purified on a short column filled with silica gel, using dichloromethane and methanol (9:1) as the eluent. The purified solvent was converted to the free base. Evaporation of the solvent gave an oily residue which was dissolved in isopropanol and the solution was diluted with hexane until the 95:5 ratio was obtained. For the separation of the enantiomers, a 10-15 mg / ml concentration of the racemic mixture was made. A mixture of hexane (plus 0.1% triethylamide) and isopropanol was used as the mobile phase.
[0105] A gradient-type mixer maintained the ratio between the solvents hexane and isopropanol at 95:5. Injection amounts were 100 .mu.l per injection. The enantiomers were collected using a fraction collector. Only fractions with a signal above 10% (10 mV) of the total scale were collected. The amounts with signals below 10% were collected separately and used for a secon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com