Production process of alpha-hydroxy organic acid cyclic ester
A manufacturing method and organic acid technology, applied in the direction of organic chemistry, can solve the problems of difficult process control, increase the complexity of the process, and reduce the temperature in the tank, so as to simplify the separation and purification steps of products, reduce the troubles of separated products, and increase the surface area Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0043] Preparation Example 1: Polymerization of Lactic Acid Oligomers:
[0044] 1000 grams of lactic acid raw material (concentration 88% by weight), which is equivalent to 880 grams of pure lactic acid, is placed in a 2-liter three-port reactor attached to a distillation device, and a small amount of nitrogen is introduced to aerate and the temperature is raised to 170° C. for 2 hours, until no more Until the water was distilled off, a lactic acid oligomer with a molecular weight of 800 was obtained.
preparation example 2
[0045] Preparation Example 2: Polymerization of Glycolic Acid Oligomers:
[0046] Glycolic acid (concentration 70%) 1000 grams and polytetramethylene glycol ether (polytetramethylene ether glycol; Dalian company PTMEG-2000) 1000 grams and antimony trioxide (Sb 2 o 3 ) of 2 grams, placed in a 2-liter three-port reactor attached to a distillation device, dehydrated under nitrogen, the dehydration temperature is 195°C, and the dehydration is until the water droplet distillation speed is very slow, the whole time takes about 2 hours, and the water is released , connected to a vacuum system, and evacuated at 195-200°C until the pressure dropped to 25mmHg, about 2 hours until no water dripped out completely, and the glycolic acid oligomer was obtained with a molecular weight of 2850.
Embodiment 1
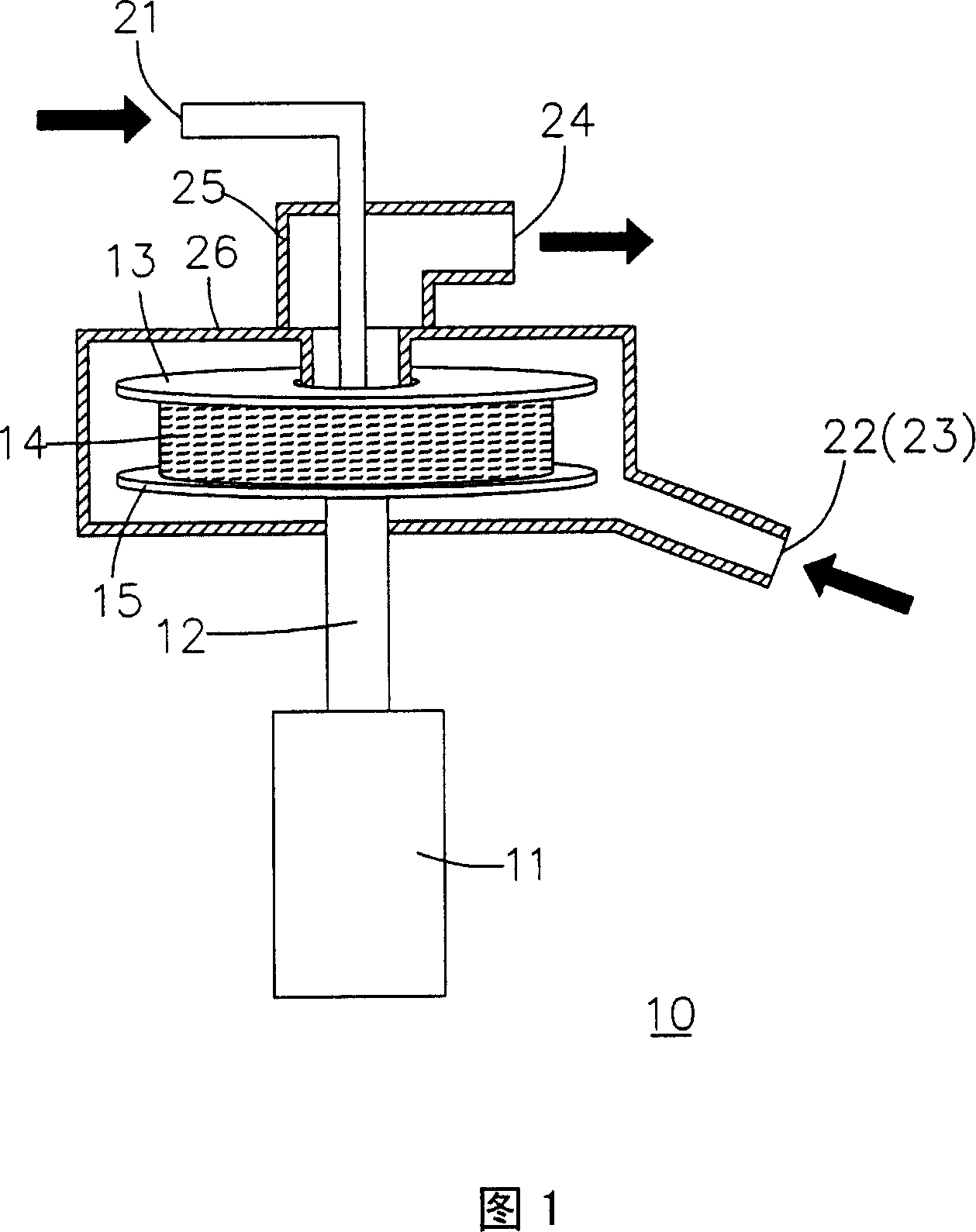
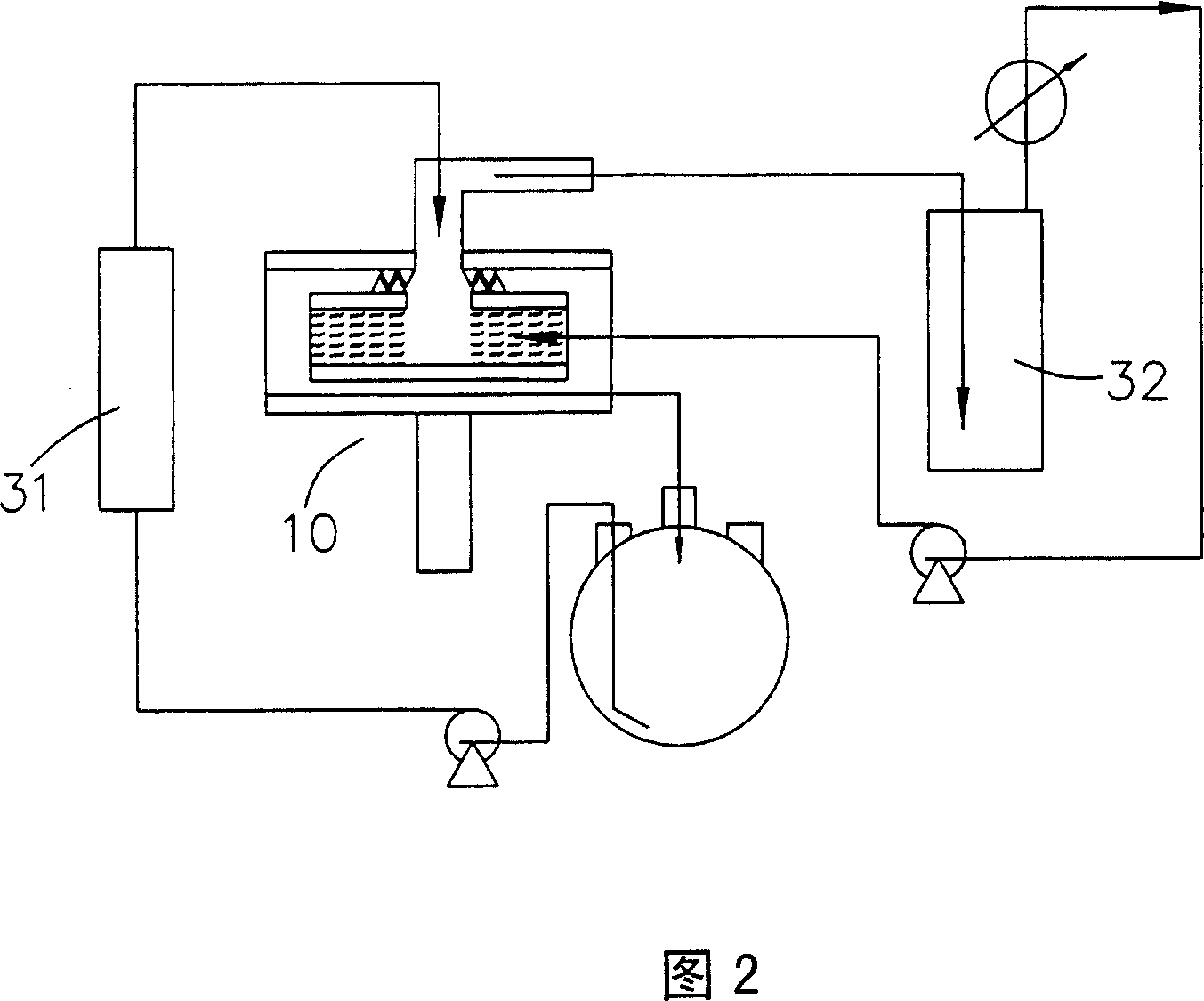
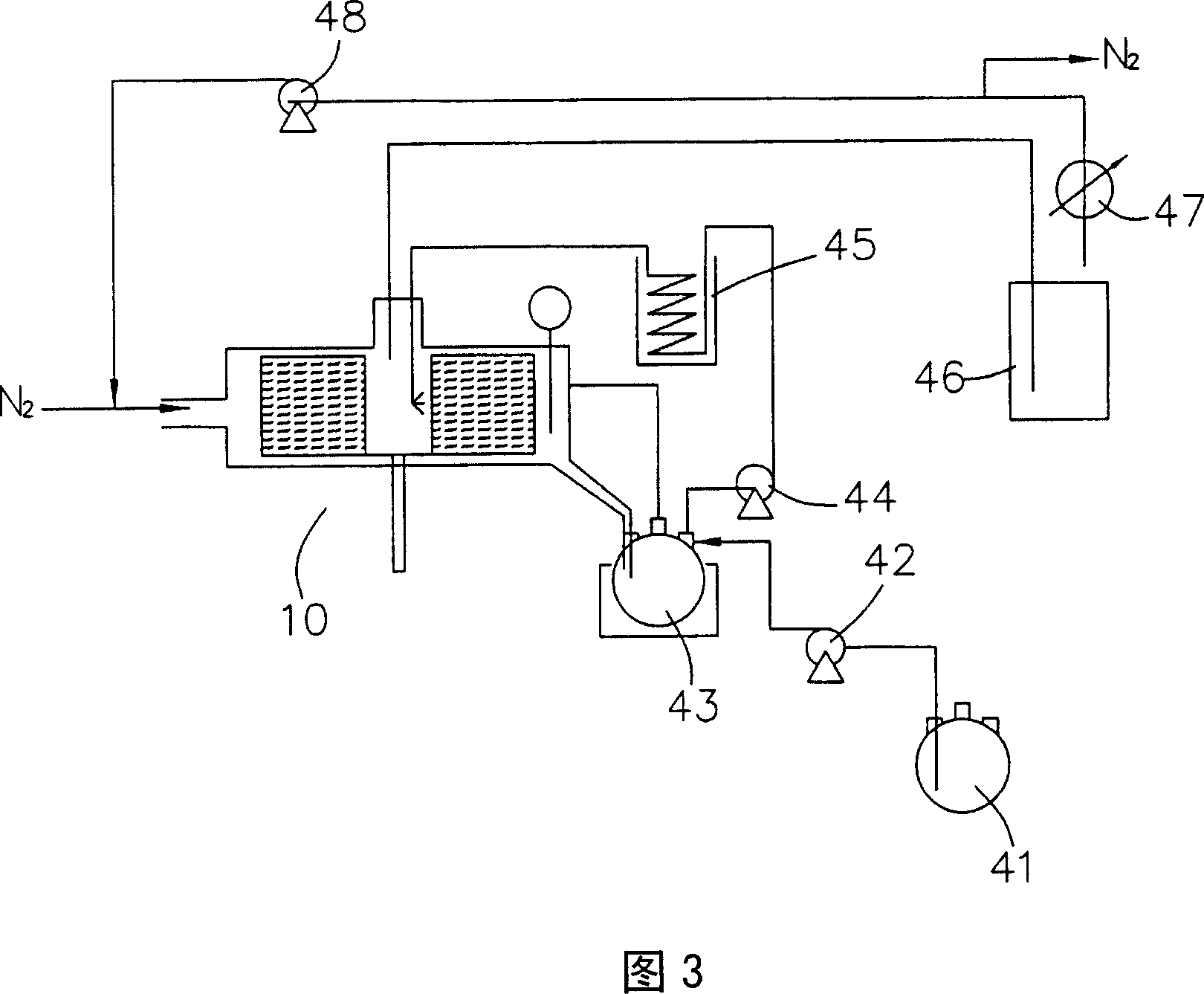
[0048]Using the batch method high gravity cracking system shown in Figure 2, the lactic acid oligomers prepared in Preparation Example 1 are added to the liquid circulation tank, and then the lactic acid oligomers are transported in batches by a pump to be heated by a heater. After the lactic acid oligomer in the vessel reaches a temperature of 190°C, most of the lactic acid oligomer is cracked into liquid lactide. Then the liquid mixture of the aforementioned lactic acid oligomer and lactide is sent into the rotating disk of the supergravity device, the rotating speed of the rotating disk is 1300rpm, and the volume flow rate of the aforementioned liquid mixture is 0.35 (L / min); in addition, a carrier gas (nitrogen) Inflow from the entrance of the supergravity device, the volume flow rate of the carrier gas is 35 (L / min), the temperature of the carrier gas is 63°C, the ratio of the volume flow rate of the gas to liquid (the volume flow rate of the carrier gas (L / min) / the volume...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com