Synthesis of polypeptide antibody from anti-human myocardial troponin I, its production and use
A technology for cardiac troponin and synthetic peptides, applied in the direction of anti-animal/human immunoglobulin, material inspection products, biological testing, etc., can solve difficulties, high extraction and purification costs, difficult to meet early diagnosis of acute myocardial infarction, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthesis of Anti-Human Cardiac Troponin I Polypeptide
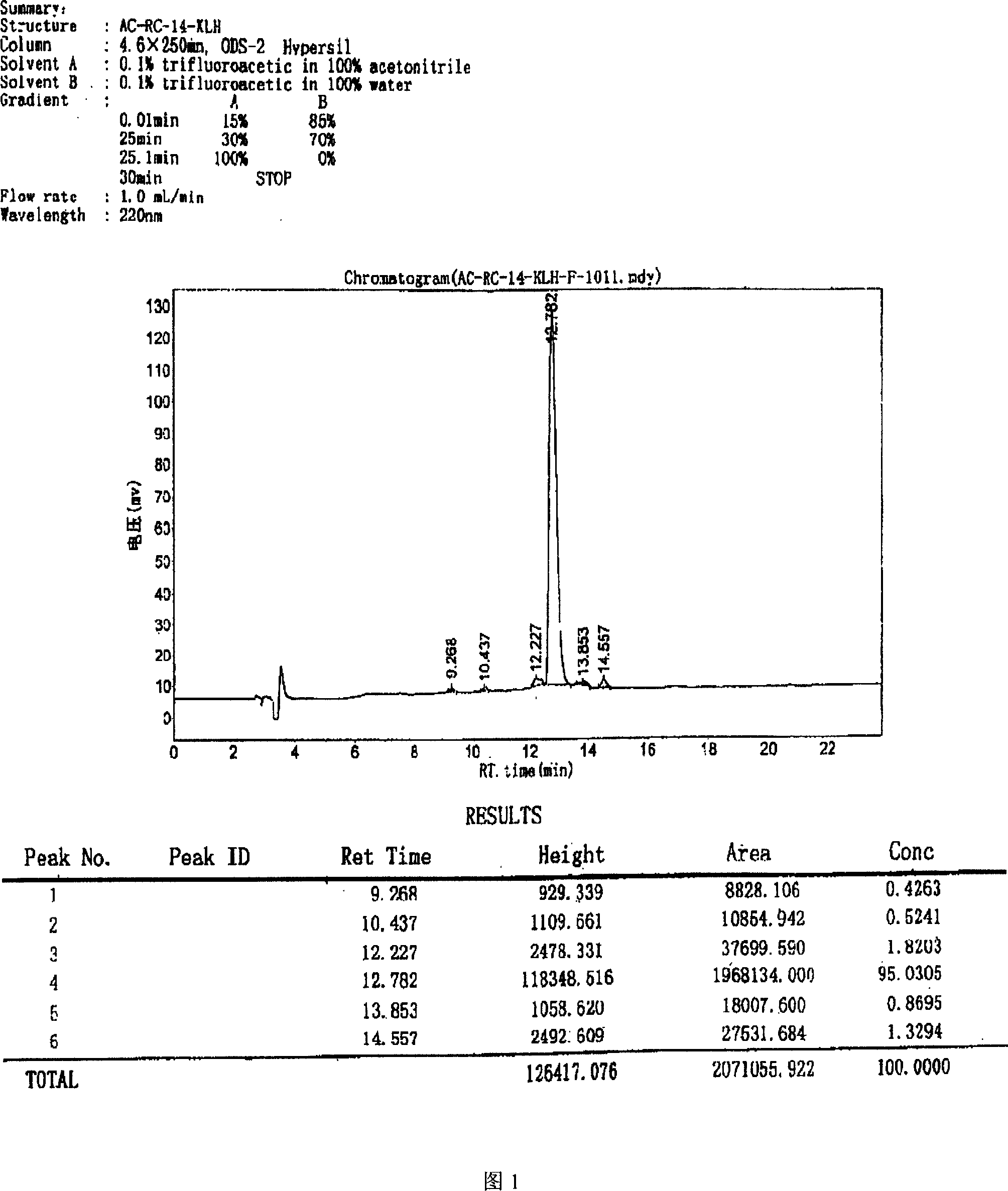
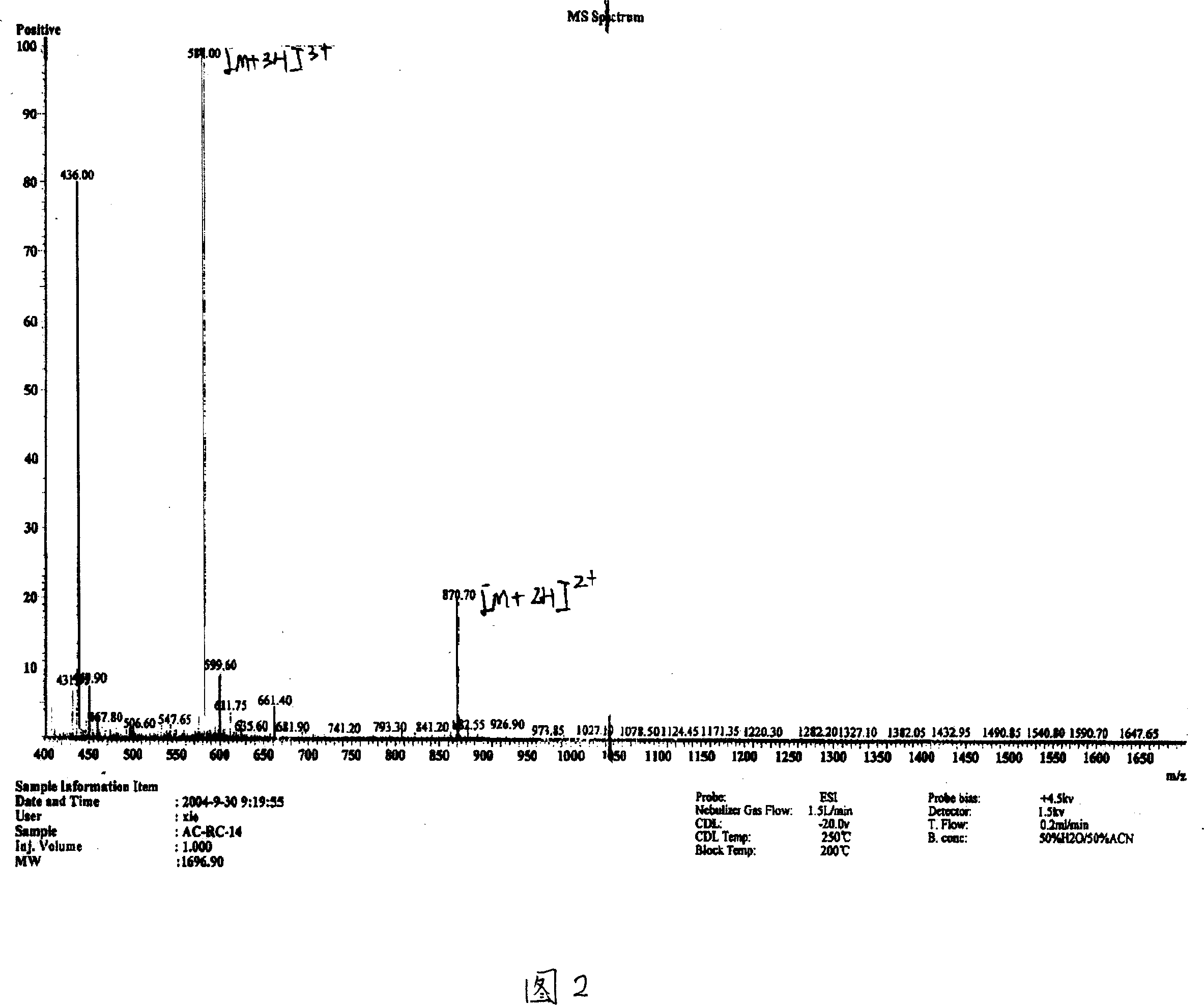
[0046] Use the Hopp&Woods model in Protscale software to predict the hydrophilicity, antigenic type and homology analysis of cTnI molecules, select the 98-110th amino acid at the N-terminal of cTnI as a polypeptide with 13 amino acids, and synthesize the peptide mainly through solid-phase peptide synthesis Method preparation. Using Fmoc-protected amino acids as raw materials, the amino acid sequence of the required peptide RQLHARVDKVDEE was synthesized in a stepwise condensation manner on a solid-phase support (resin). Finally, the peptide is "cleaved" from the carrier by an appropriate method, and all protective groups are removed. The polypeptide can be purified up to 95% by high performance liquid chromatography (see Figure 1 for details), and a pure synthetic peptide antigen can be obtained.
Embodiment 2
[0048] Ligation of synthetic peptides to carrier protein (KLH) (MBS ligation method)
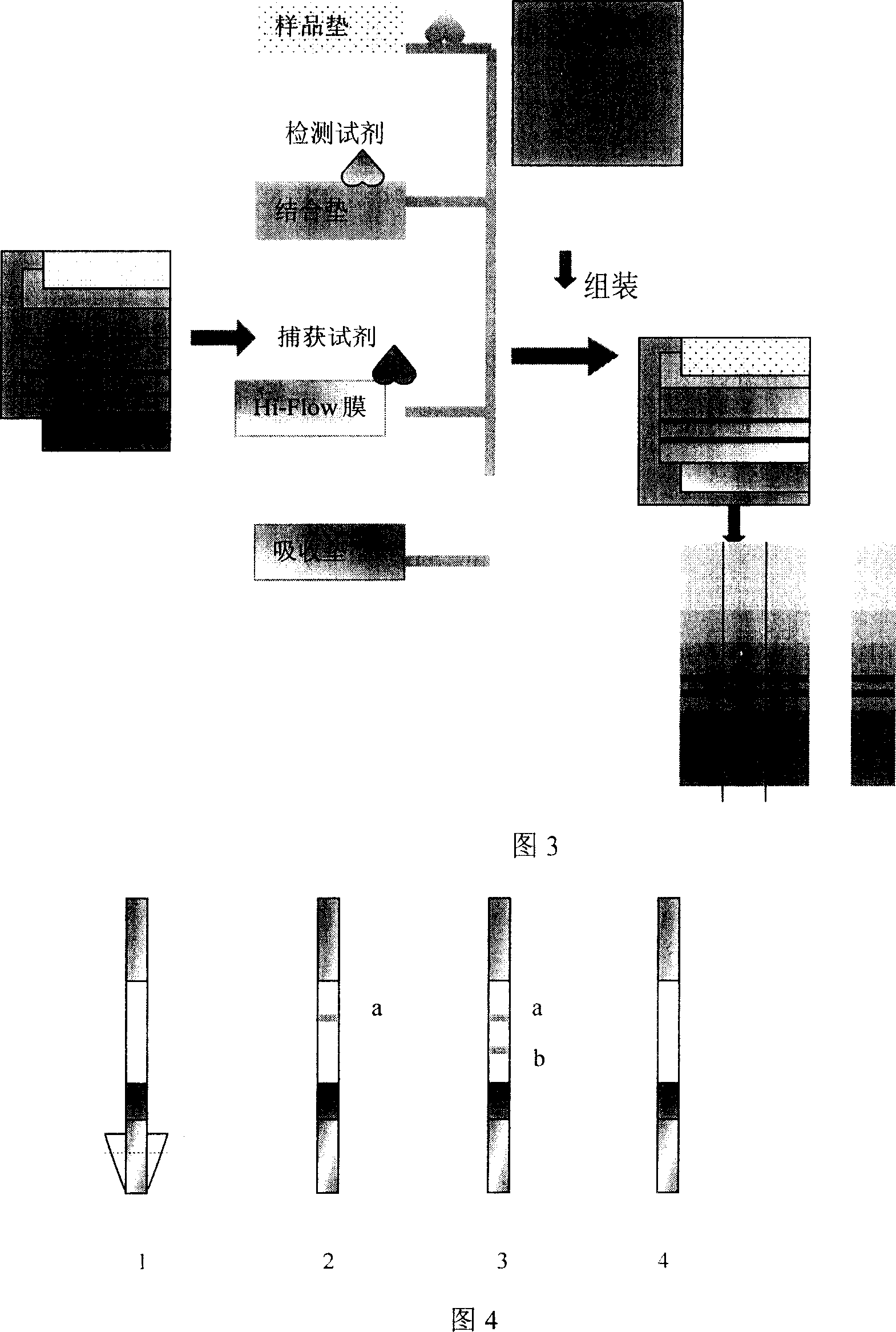
[0049] (1) Dissolve 5 mg of KLH in 0.5 ml of PBS, place in a dialysis bag, dialyze with PBS overnight at 4°C, and then transfer to a glass test tube. (2) Add 70ul of MBS / DMF and stir at room temperature for 30 minutes. (3) Take the PD-10 chromatographic column pre-equilibrated with PB, add the sample slowly, and elute with PB, collect about 12-20 tubes, each tube is 0.5ml, and measure the 280nm OD value of the eluent by a UV-visible spectrophotometer. The first peak represents the MBS / KLH conjugate, which is flocculent; the second peak contains free MBS. The MBS / KLH conjugate fractions were pooled. (4) Dissolve 5mg of synthetic peptide in PBS, if the synthetic peptide is insoluble in PBS, it can be dissolved in 6mol / 0.1mol / lPB. (5) Mix the synthetic peptide solution with the MBS / KLH conjugate, adjust the pH to 7.3 with 0.1mol / l hydrochloric acid or 0.1mol / l sodium hydroxide, stir magnetic...
Embodiment 3
[0051] Preparation of Polyclonal Antibody to cTnI Synthetic Peptide Antigen
[0052] The specific process of polyclonal antibody preparation technology includes: animal immunization, antiserum titer detection, harvesting of antiserum, antibody purification and antibody quality identification, which are described as follows:
[0053] 1. Animal immunization: New Zealand big-eared white rabbits weighing 1.5-1.8Kg were selected, and human cTnI polypeptide synthetic antibodies were prepared after initial and multiple booster immunizations;
[0054] (1). Detection of immune effect: 10-14 days after the second booster immunization, a small amount of blood was collected from the rabbit's ear vein, the serum was separated, and the antibody titer in the rabbit serum was detected by indirect ELISA method.
[0055] (2). Collection of antiserum: a large number of bloodletting at one time. When the titer of antiserum reaches the maximum and no longer increases (or begins to decline), the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com