Nickel oxyhydroxide, manufacturing method therefor, and alkaline primary battery
A nickel oxyhydroxide, nickel hydroxide technology, applied in nickel oxide/nickel hydroxide, alkaline storage battery electrodes, electrodes of primary batteries, etc., can solve problems such as difficulties and oxidation difficulties, and achieve the effect of improving pulse discharge performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
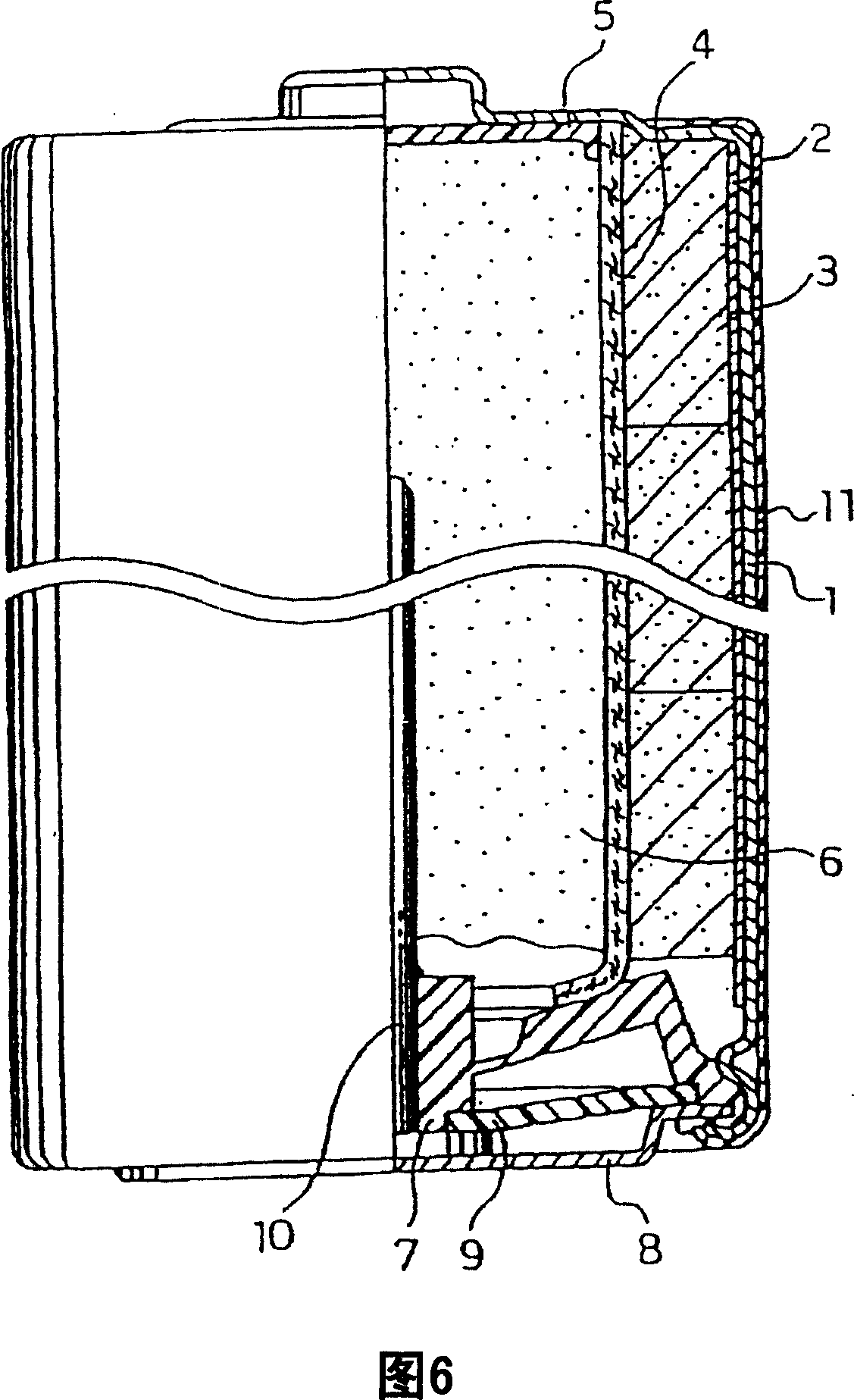
Image
Examples
Embodiment 1
[0103] (1) Synthesis of nickel hydroxide
[0104] A 2L aqueous solution containing 2mol / L ammonia and 0.05mol / L ammonium sulfate was put into a reaction vessel (2L) with a stirring blade, an oxygen nozzle, a pH electrode, a voltage test electrode, and a thermometer. Add 25% by weight of ammonia water and stir appropriately so that the pH value of the aqueous solution is maintained at 10.5, and the temperature under ambient pressure is maintained at 50°C. A linear nickel powder obtained by pyrolysis of nickel carbonyl (255 type: manufactured by INCO) was added to the aqueous solution in an amount of 250 g to obtain a suspension. In this step, nickel is activated. From the moment when the oxidation-reduction potential of the above suspension reached about -600 mV (compared to SCE), oxygen (50 mL / min) was supplied from the oxygen nozzle. Oxygen supply was performed for 15 hours to obtain a slurry containing nickel hydroxide. Use a magnet to remove unreacted metallic nickel powder fro...
Embodiment 2
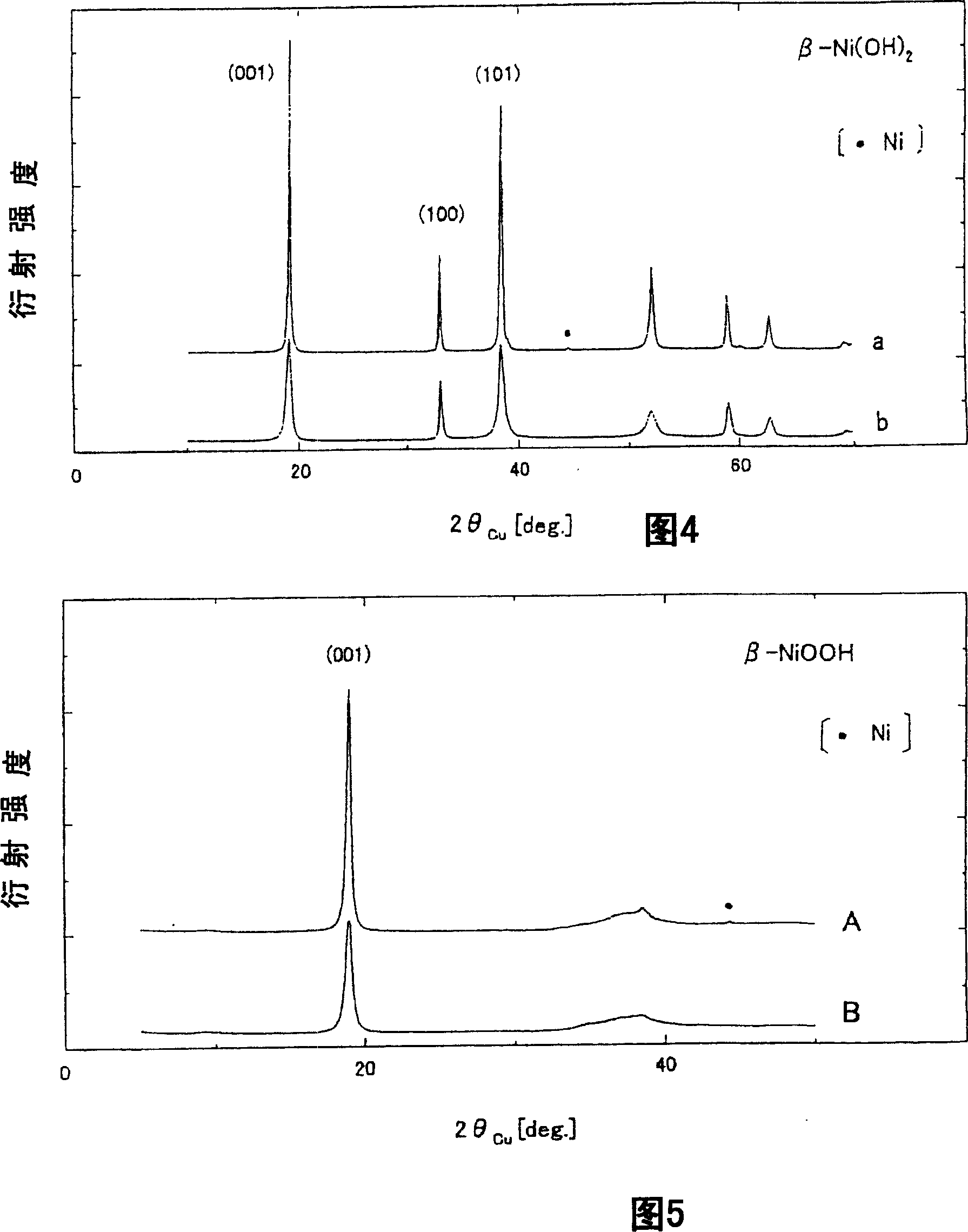
[0138] The crystallinity, average particle size and metallic nickel content of nickel oxyhydroxide were evaluated.
[0139] The nickel hydroxide "a" prepared in Example 1 in an amount of 5 kg was put into a 0.1 mol / L sodium hydroxide aqueous solution in an amount of 20 L. 1.5 equivalents of sodium hypochlorite aqueous solution (concentration of available chlorine: 10% by weight) as an oxidizing agent was added and stirred to be converted into nickel oxyhydroxide. At this time, the reaction environment temperature was set to 50°C, and the treatment time after the sodium hypochlorite aqueous solution was put in was set to 6 hours. The obtained particles were thoroughly washed with water and filtered, and they were used as nickel oxyhydroxide slurry A1. The nickel oxyhydroxide paste A1 is a product of substantially the same magnification as the nickel oxyhydroxide A prepared in Example 1.
[0140] 5 kg of nickel hydroxide "a" was put into 20L of 1mol / L sodium hydroxide aqueous soluti...
Embodiment 3
[0154] When nickel oxyhydroxide is a solid solution containing metal elements (such as cobalt and manganese), examples are introduced to observe the effect.
[0155] A solid solution nickel hydroxide c1 containing cobalt was obtained in the same manner as in Example 1, except that metal cobalt powder was added when the nickel powder was added. The amount added is set so that the content of cobalt is 0.05 mol% with respect to the total amount of metal elements contained in nickel hydroxide. Similarly, except for adding cobalt so that the cobalt content relative to the total amount of metal elements contained in nickel hydroxide is 0.1 mol%, 1 mol%, 3 mol%, 7 mol%, 10 mol%, and 12 mol% , The nickel hydroxide c2 to c7 were obtained in the same manner as above.
[0156] Also, except for using metal manganese powder (a reagent produced by Sigma-Aldrich) instead of metal cobalt powder, nickel hydroxide d1 to d7 were prepared in the same manner as above, that is, containing manganese and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com