Candesartan Cilexetil and its precursor compound preparation method
The technology of a precursor compound, candesartan cilexetil, is applied in the direction of organic chemistry, which can solve the problems of difficult preparation, unsatisfactory yield and complex structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
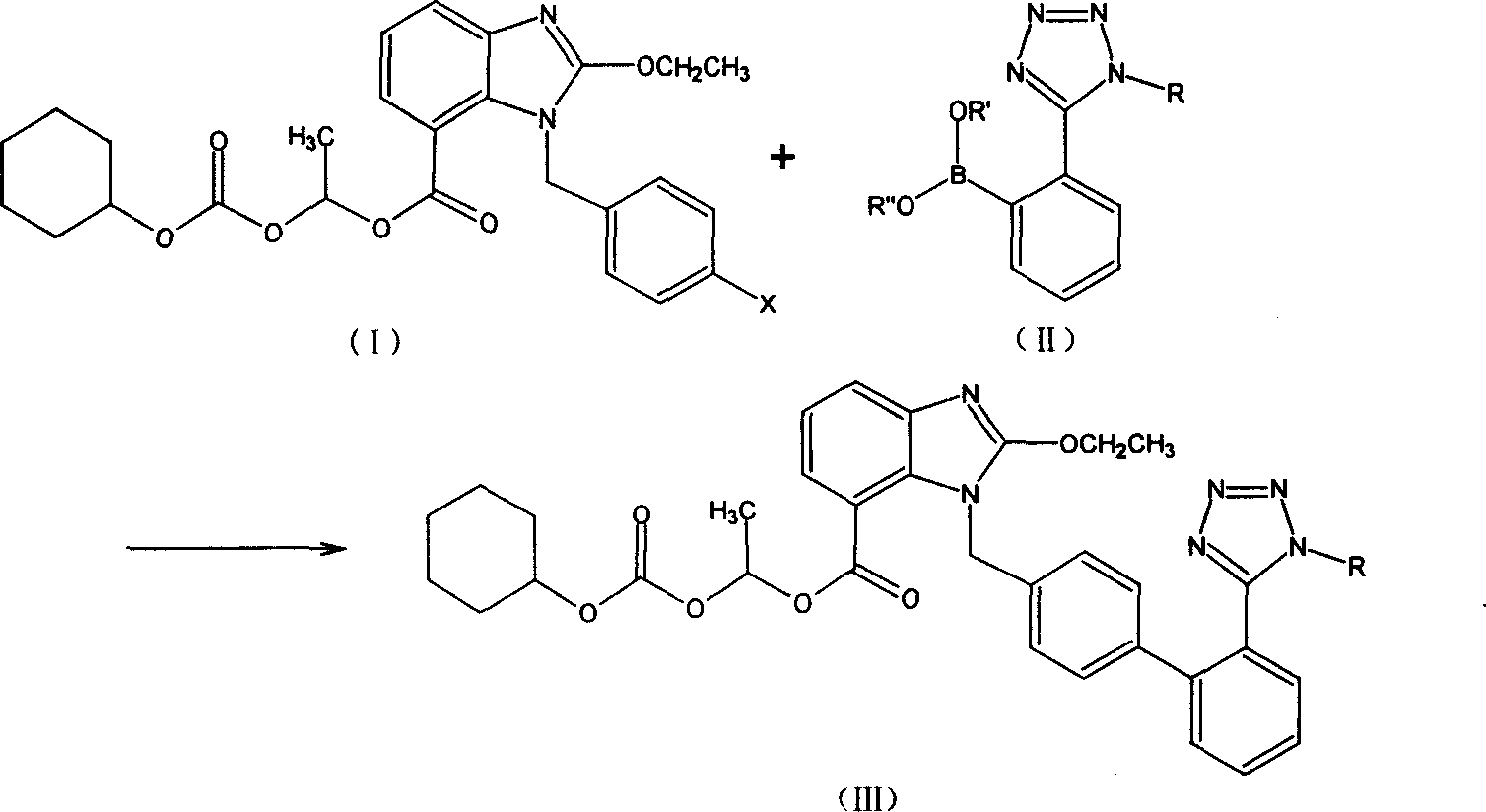
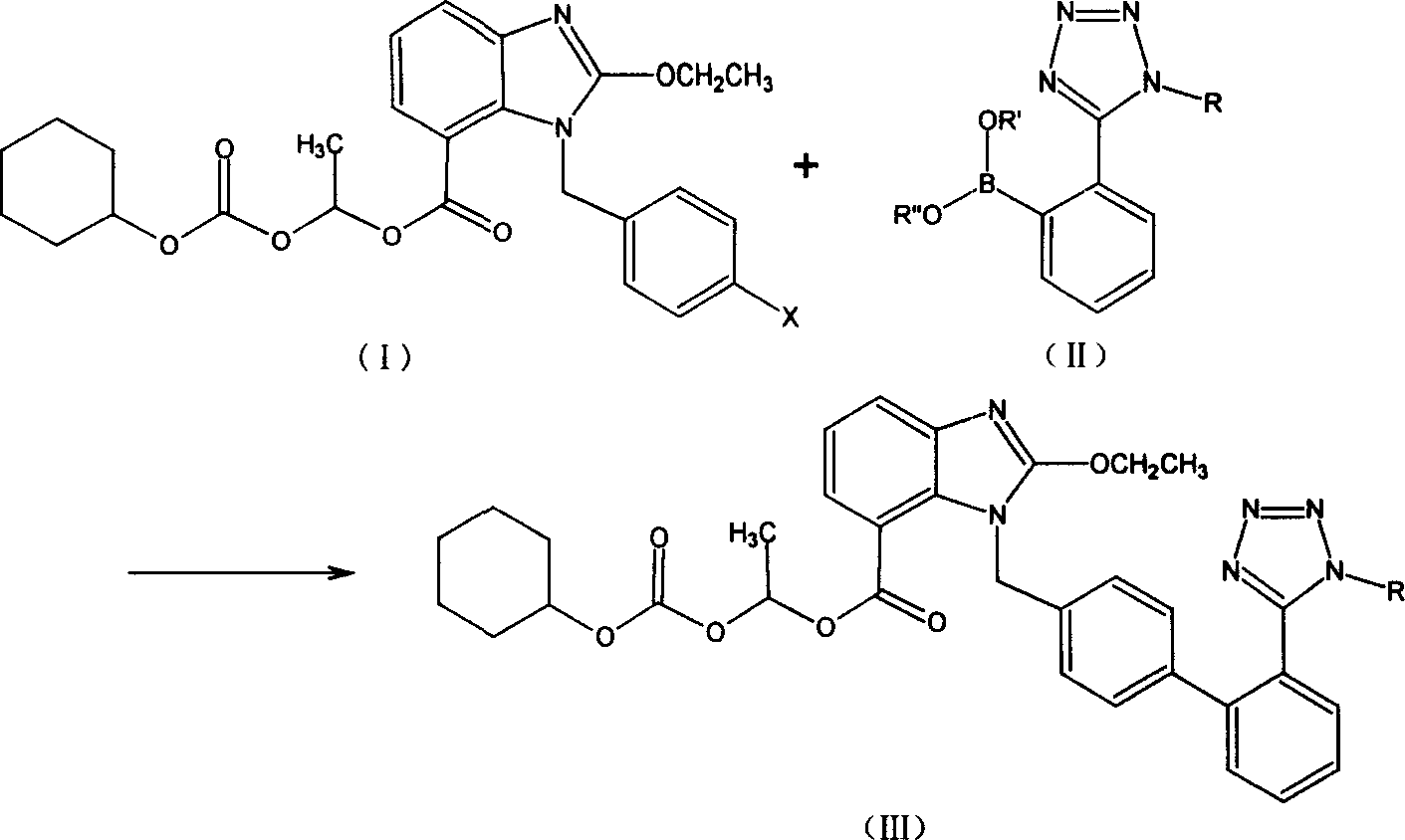
[0026] 2-Ethoxy-1-[[2′-(trityltetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1H-benzimidazole- Preparation of 1-(cyclohexyloxycarbonyloxy)ethyl 7-carboxylate (III)
[0027] Put 80 parts of the mixed solvent of DME / THF at 1 / 1 (volume) into the reactor, pour in nitrogen to drive out the air in the system, and put in 1 part of triphenylphosphine (Ph3P) and Pd(OAc)2 0.2 After stirring for 30 minutes at room temperature, 10 parts of 2-(1-trityl-1H-tetrazol-5-yl)phenylboronic acid (II) were added, and stirring was continued for 10 minutes at room temperature. Join K in turn 2 CO 3 6 parts of fine powder, 2-ethoxy-1-(4'-bromophenyl)methyl-1H-benzimidazole-7-carboxylic acid-1-[[(cyclohexyloxy)carbonyl]oxy]ethane 12 parts of ester (I), heated to about 75° C. and refluxed for 15 hours (thin layer chromatography monitoring: Hex / EtOAc 2:1).
[0028] After cooling to normal temperature and suction filtration, the filtrate can be distilled under reduced pressure to remove most of the solvent. 80 ml...
Embodiment 2
[0030] 2-Ethoxy-1-[[2′-(trityltetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1H-benzimidazole- Preparation of 1-(cyclohexyloxycarbonyloxy)ethyl 7-carboxylate (III)
[0031] Put 80 parts of dioxane into the reactor, blow in nitrogen to remove the air in the system, put in 0.2 parts of triphenylphosphine (Ph3P) and 0.5 parts of Pb[Ph3]4 in turn, stir at room temperature for 30 minutes, and then add 2 -(1-Trityl-1H-tetrazol-5-yl) phenylboronic acid ethyl ester (II) 10 parts, and continue to stir at room temperature for 10 minutes. Sequentially add 6 parts of K2CO3 fine powder, 2-ethoxy-1-(4'-bromophenyl)methyl-1H-benzimidazole-7-carboxylic acid-1-[[(cyclohexyloxy)carbonyl] 12 parts of oxy]ethyl ester (I) were heated to 90° C. and the reaction was incubated for 6 hours (thin layer chromatography monitoring: Hex / EtOAc=2 / 1).
[0032] Cool to room temperature and filter with suction. The filtrate can be distilled under reduced pressure to remove most of the solvent. Add 80ml of toluene and 8...
Embodiment 3
[0034] Preparation of candesartan cilexetil (IV)
[0035] The 2-ethoxy-1-[[2'-(trityltetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]- 10g of 1H-benzimidazole-7-carboxylic acid 1-(cyclohexyloxycarbonyloxy)ethyl (III), dissolved in 80ml of acetone, adjusted to pH 2 with 5% hydrochloric acid at room temperature, and stirred for 5-10 hours , Monitoring the progress of the reaction by means of thin layer chromatography (thin layer chromatography monitoring: chloroform: petroleum ether: methanol = 4:3:1) or high performance liquid chromatography.
[0036] Then, 5% NaOH aqueous solution was slowly added dropwise to the reactant until it became neutral, most of the acetone was removed by distillation under reduced pressure, 50 ml of ethyl acetate was added, and the mixture was cooled and crystallized, and the mixture was stirred for 30 minutes and then filtered. The filter cake was washed with an appropriate amount of ice ethyl acetate, purified in DMF / water, or purified by silica gel column c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com