UV spectrophotometry and HPLC combined method for determining content of phenylpropanol
A technology of high performance liquid chromatography and determination method, which is applied in the field of combined determination of phenylpropanol content by ultraviolet spectrophotometry and high performance liquid chromatography, and can solve the problem of inappropriate selection of buffer solution to stabilize the acidity of medium and detection wavelength and ineffective application. Measurement, not considering, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
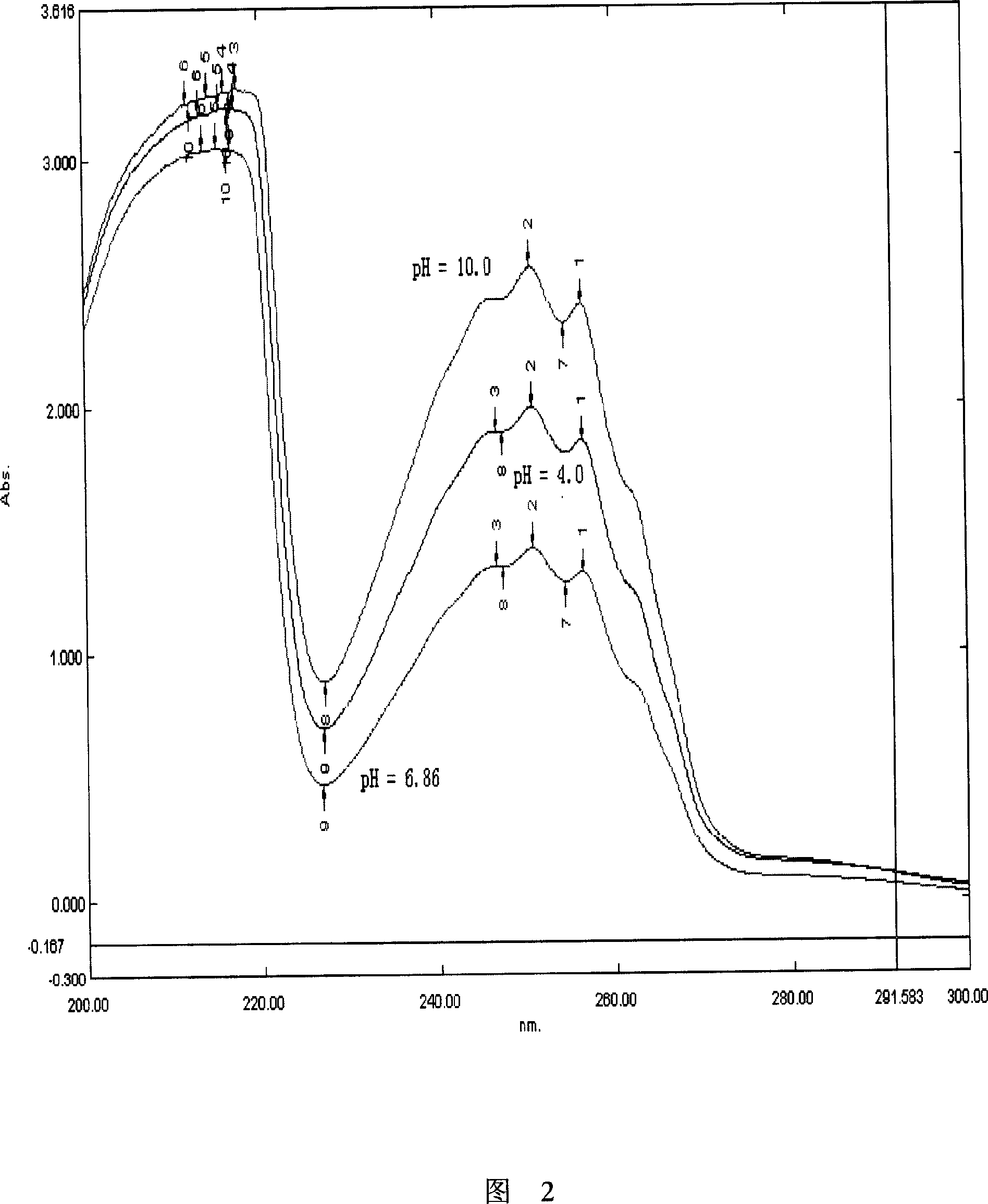
[0102] Example 1: Accurately weigh 110 mg of phenylpropanol and 935 mg of β-CD (1:1 mol) into a 100 ml volumetric flask, add 0.1% phosphate buffer with pH=6.86 to constant volume, and ultrasonic treatment for 30 minutes, which is 1.10 mg / ml Standard solution of phenylpropanol solution. Dilute by 20, 10, 5, 2.5, and 2 times, and take the average of three measurements at each point to obtain the standard curve of phenylpropanol under the condition of β-CD containing the prescribed dose: A = 1.5256C + 0.0656, r = 0.9999.
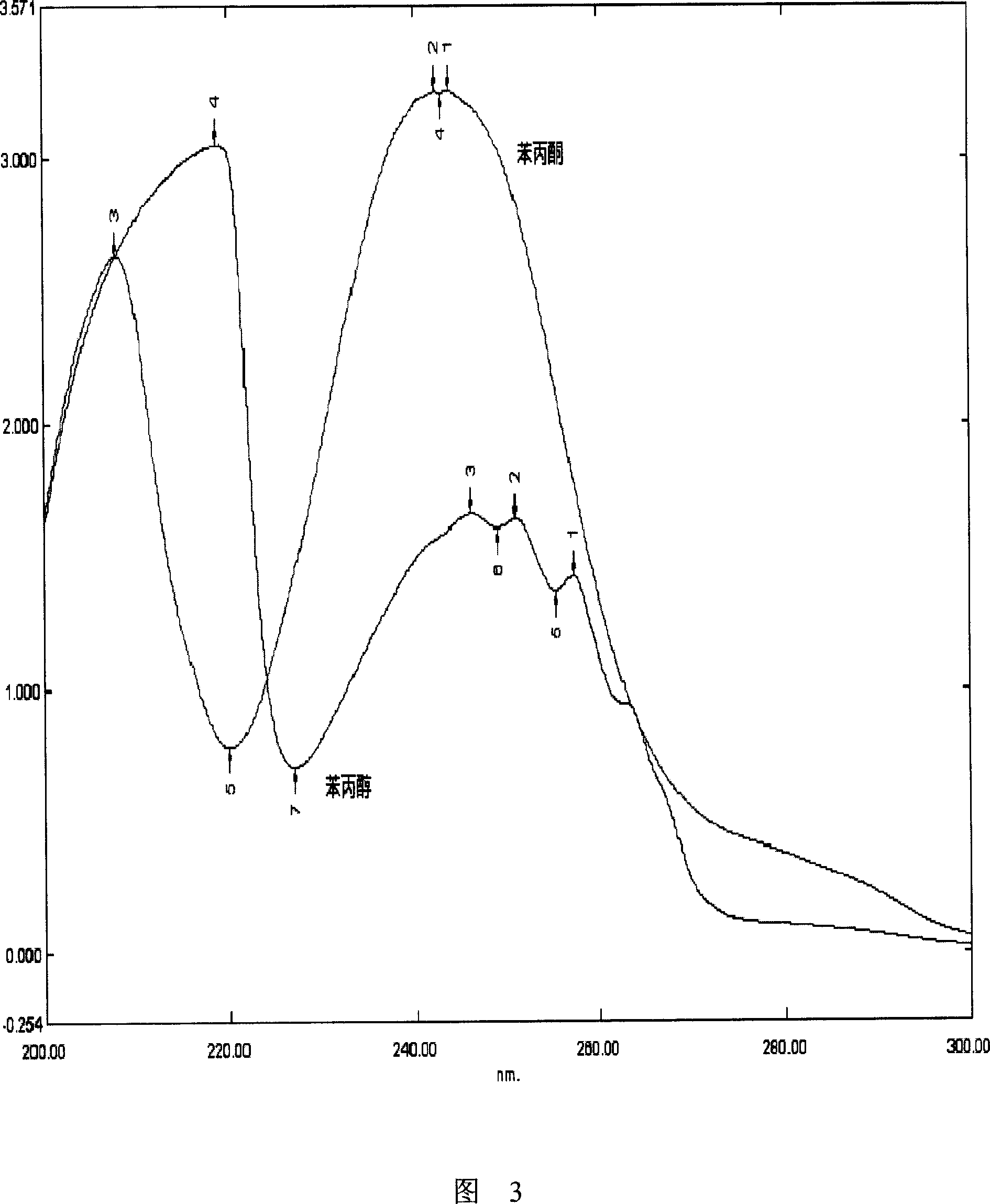
[0103] Take phenylpropanol tablets (batch number 050516), crush into fine powder, accurately weigh 65mg, sonicate with 20ml of phosphate buffer solution for 30min, dilute to 25ml, and prepare a solution containing about 0.26mg / ml of phenylpropanol at 257nm The absorbance was measured, A = 0.4620, and the calculated content of phenylpropanol was 99.55% (labeled amount in phenylpropanol tablets).
Embodiment 2
[0104] Example 2: Basically the same as Example 1, but adding 0.5% acetic acid-sodium acetate buffer with pH=4.02 to make the volume constant to obtain a standard curve of phenylpropanol under the condition of β-CD containing the prescribed dose: A=1.4996C+0.1733, r = 0.9987. The absorbance was measured at 257nm, A=0.5632, and the calculated content of phenylpropanol was 99.61%.
[0105] Determination
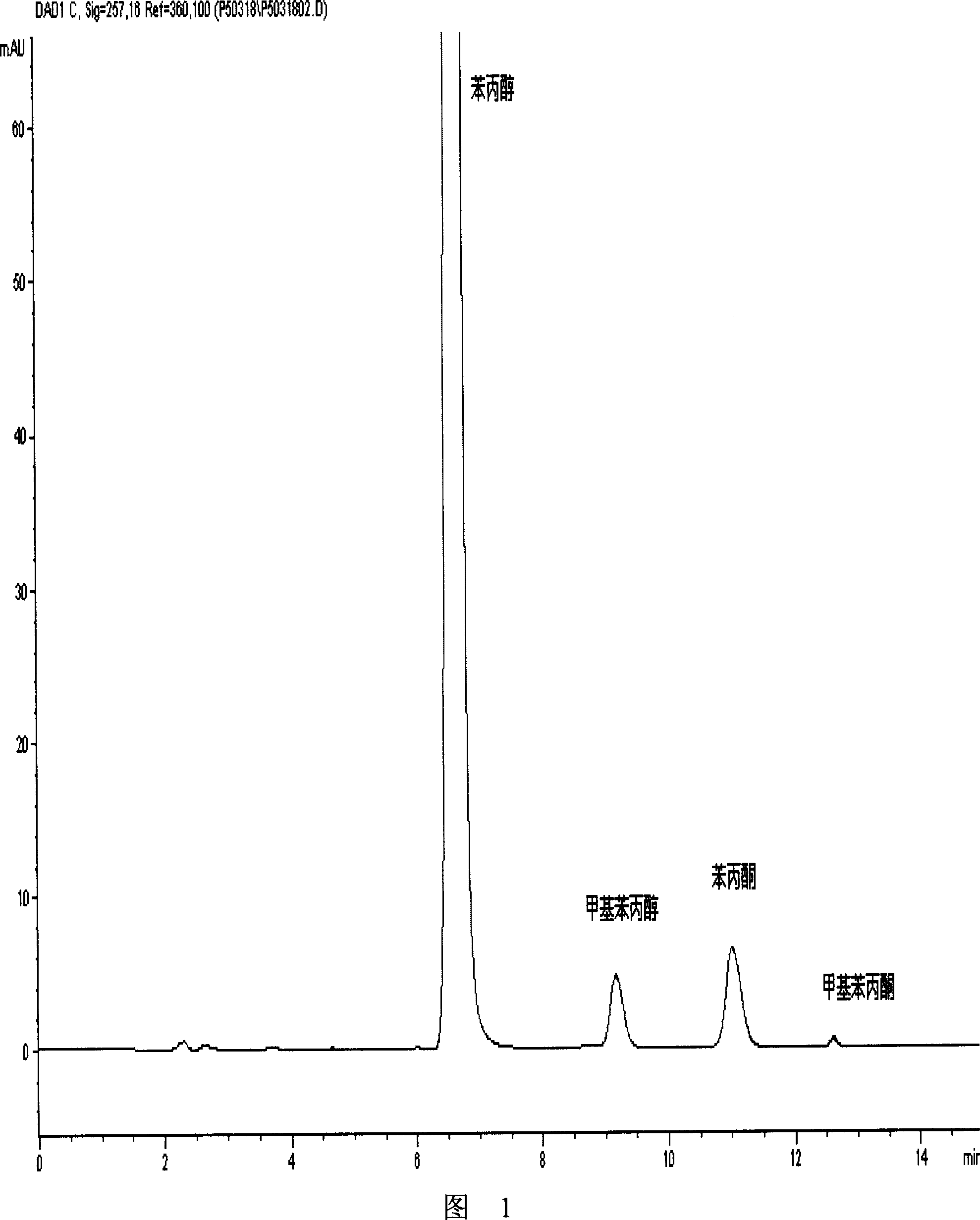
[0106] The peak area ratio of phenylacetone to phenylpropanol=0.28, which is less than 1.98 (the content of phenylacetone is 0.99%). The content of phenylacetone in batch number 050516 phenylpropanol tablets is less than 1%, and the sample is qualified.
[0107] Determination
[0108] The peak area ratio of phenylacetone to phenylpropanol=0.30, which is less than 2.33 (the content of phenylacetone is 0.99%). The content of phenylacetone in batch number 050516 phenylpropanol tablets is less than 1%, and the sample is qualified.
Embodiment 5
[0109] Example 5: Basically the same as Example 2, but adding 0.5% phosphate buffer solution with pH=5.00 to make the volume constant.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com