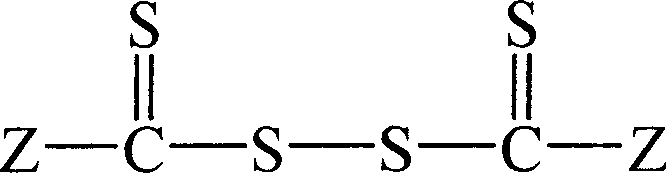
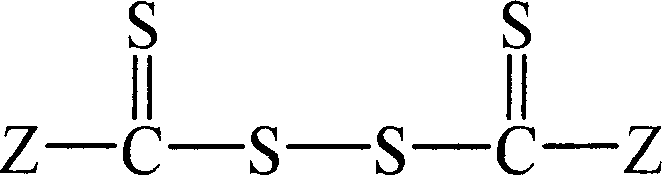Preparation method of acrylic nitrile homopolymer (copolymer)
A technology of acrylonitrile and copolymer, which is applied in the field of preparation of acrylonitrile homopolymer to achieve the effect of high yield and easy storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1 acrylonitrile homopolymer
[0028] The first step preparation of dithiobenzoyl dithio
[0029] 12.6 grams of benzyl chloride were added dropwise to the system containing 6.4 grams of sulfur, 10 grams of sodium methylate, and 70 grams of methanol within one hour using a dropping funnel. The resulting brown solution was heated to 80° C. and refluxed overnight. After cooling to room temperature, the mixture was filtered to remove white sodium chloride solid, and then methanol was removed on a rotary evaporator. The resulting solid was redissolved in 100 ml of distilled water and washed three times with ether (3 x 50 ml). Add 50ml of diethyl ether to the solution, acidify the biphasic mixture with 30% hydrochloric acid until the aqueous layer loses its characteristic brown color and the upper layer appears dark purple, separate the ether layer solution, add 120ml of distilled water, and extract disulfide with 240ml of 30% sodium hydroxide s...
Embodiment 2
[0034] The preparation of embodiment 2 acrylonitrile homopolymer
[0035] The first step preparation of dithiophenylacetyl dithio
[0036] In a three-neck flask with a reflux condenser and a dropping funnel, under nitrogen protection, add 7.7 grams of magnesium chips, 150 ml of anhydrous ether, and drop 40 grams of benzyl chloride into the system within 30 minutes under an ice-water bath , continued to stir for 1 hour, and then refluxed for 0.5 hours. After cooling, 24 g of carbon disulfide was added dropwise within 15 minutes at 0° C., and the reaction was continued for 3 hours. The mixture was poured into 150 ml of ice water, the organic layer was separated, and the aqueous layer was washed three times with ether (3 x 50 ml). The aqueous layer was acidified with 30% hydrochloric acid and extracted with ether until it became colorless, and the ether layer solutions were combined. 100 ml of distilled water was added, extracted with 30% sodium hydroxide solution, and washed ...
Embodiment 3
[0039] The preparation of embodiment 3 acrylonitrile homopolymer
[0040] With 2.5mg of azobisisobutyronitrile, 5.1mg of dithiophenylacetyl disulfide replaces azobisisobutyronitrile and dithiophenylacetyl dithio in Example 2, and others are the same as in Example 2, 0.5 g of acrylonitrile homopolymer was obtained, and the monomer conversion rate was 61.7%. As determined by GPC, the number average molecular weight of the acrylonitrile homopolymer was 212,000, and the molecular weight distribution was 1.72.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com