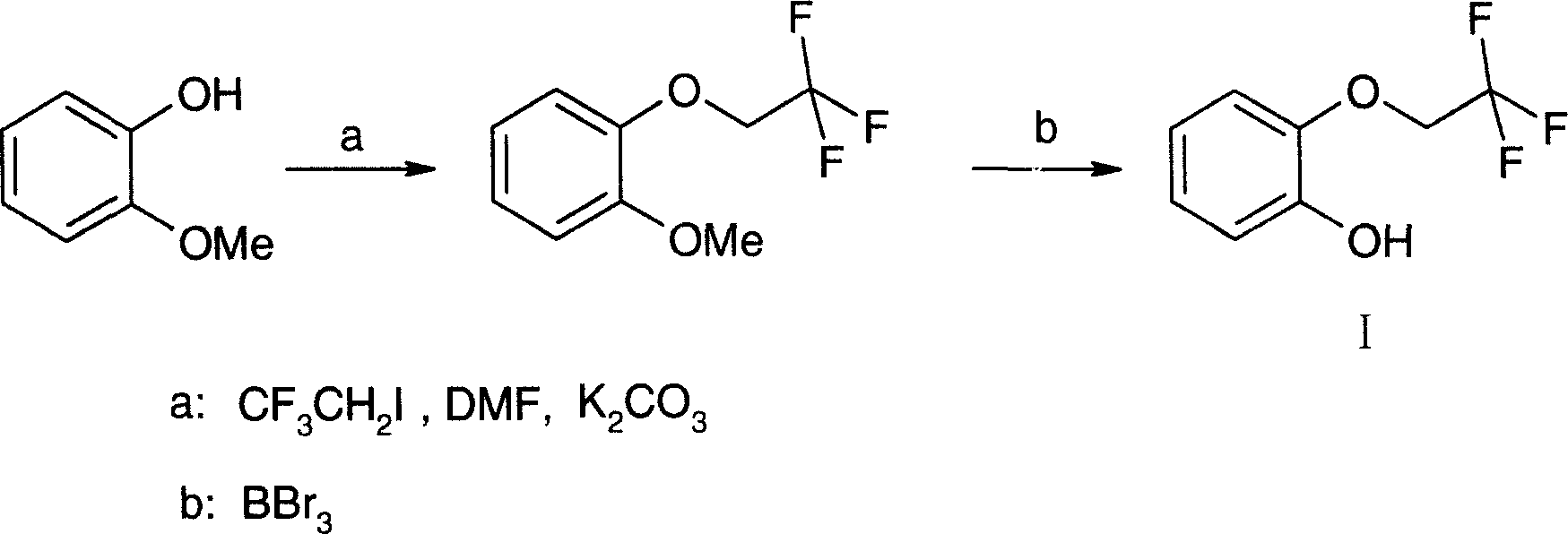
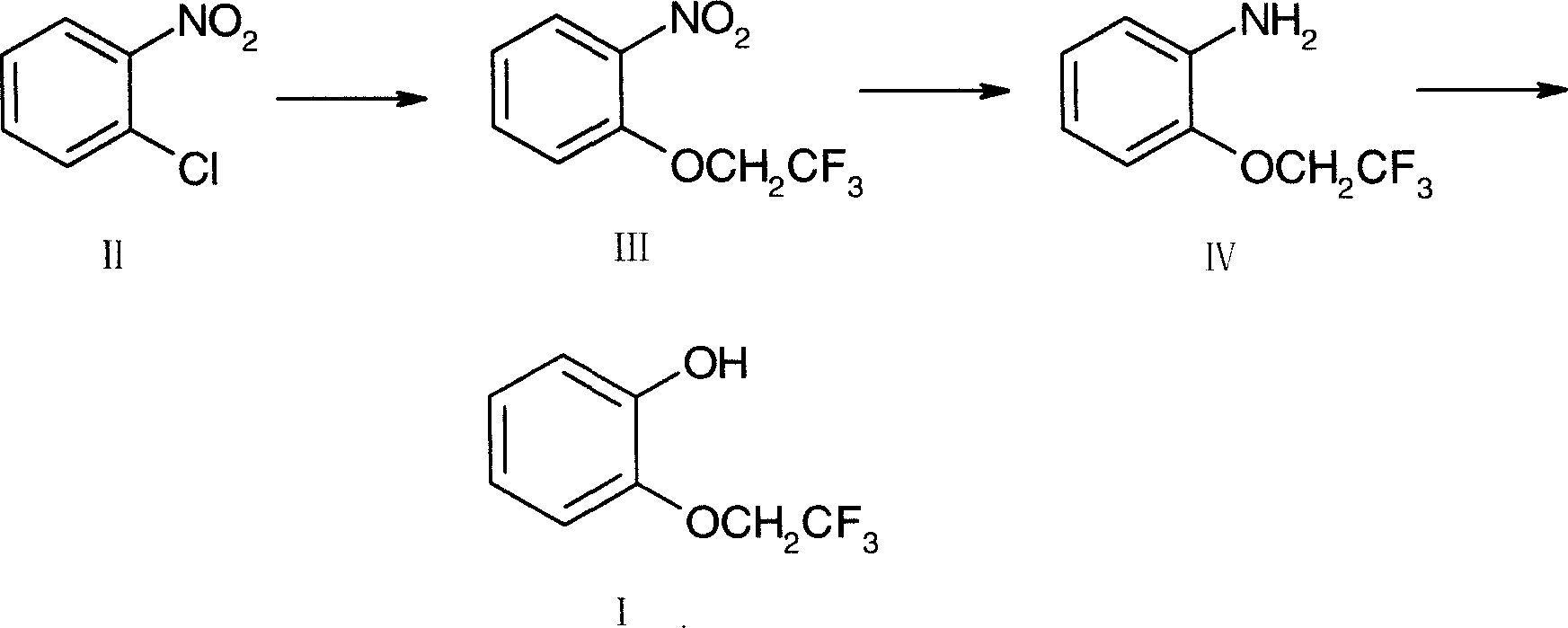
Method for preparing 2-(2,2,2-trifluoroethoxy)phenol
A technology of trifluoroethoxy and trifluoroethanol, applied in 2-(2, can solve the problems of harsh reaction conditions, easy decomposition, strong corrosion, etc., and achieve the effect of being suitable for industrial production, optimizing reaction conditions, and simplifying reaction operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1, the preparation of 2-(2,2,2-trifluoroethoxy) nitrobenzene (III):
[0017] Add o-nitrochlorobenzene (II) 100g, 50% sodium hydroxide 260g, phase transfer catalyst tetrabutylammonium bromide 8g in the reaction bottle, stir, heat up to 60 ° C, drop 2,2,2-trifluoroethanol 68g, dropwise, stirred and reacted at 70°C for 6 hours, cooled, filtered with suction, washed with water, and dried to obtain 124g of orange-red solid (III), mp 53-5°C, yield 88.4%.
[0018] 1 HNMR (CDCL 3 )δ: 4.50 (q, 2H, -CH 2 CF 3 ), 7.13-7.90 (m, 4H, Ar-H)
Embodiment 2
[0019] The preparation of embodiment 2,2-(2,2,2-trifluoroethoxy)aniline (IV):
[0020] Add 120 g of 2-(2,2,2-trifluoroethoxy) nitrobenzene (III), 1.5 L of absolute ethanol, and 10 g of 10% palladium carbon into the hydrogenation kettle, hydrogenate at normal pressure, stir the reaction at room temperature for 12 hours, pump After filtration, the filtrate was concentrated under reduced pressure to obtain 95 g of light yellow solid (IV), mp58-60°C, yield 91.6%.
[0021] 1 HNMR (CDCL 3 )δ: 3.84 (brs, 2H, -NH 2 ), 4.42 (q, 2H, -CH 2 CF 3 ), 6.70-7.26 (m, 4H, Ar-H)
Embodiment 3
[0022] The preparation of embodiment 3,2-(2,2,2-trifluoroethoxy)phenol (I):
[0023] Add 20% (W / W) sulfuric acid 210g in the reaction flask, cool in an ice-water bath, stir, add 2-(2,2,2-trifluoroethoxy)aniline (IV) 80g, react for 30 minutes, add dropwise A solution made of 30g of sodium nitrate and 50g of water, after dripping, reacted at 0-5°C for 2 hours, then frozen for later use.
[0024] Add 200g of 20% (W / W) sulfuric acid, 100g of sodium sulfate, and 500ml of toluene into the reaction flask, stir, heat to 70°C, add the above-mentioned diazonium salt solution dropwise, and continue the reaction for 2 hours, cool, and separate the liquids. Extract the aqueous phase with 200ml×2 toluene, combine the toluene layers, wash with water, concentrate to 1 / 3 volume, extract with 30% (W / W) sodium hydroxide 200g×3, combine the aqueous layers, acidify with 2N hydrochloric acid, and add 200ml×2 toluene Extract and combine the toluene layers, dry over anhydrous sodium sulfate, filter,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com