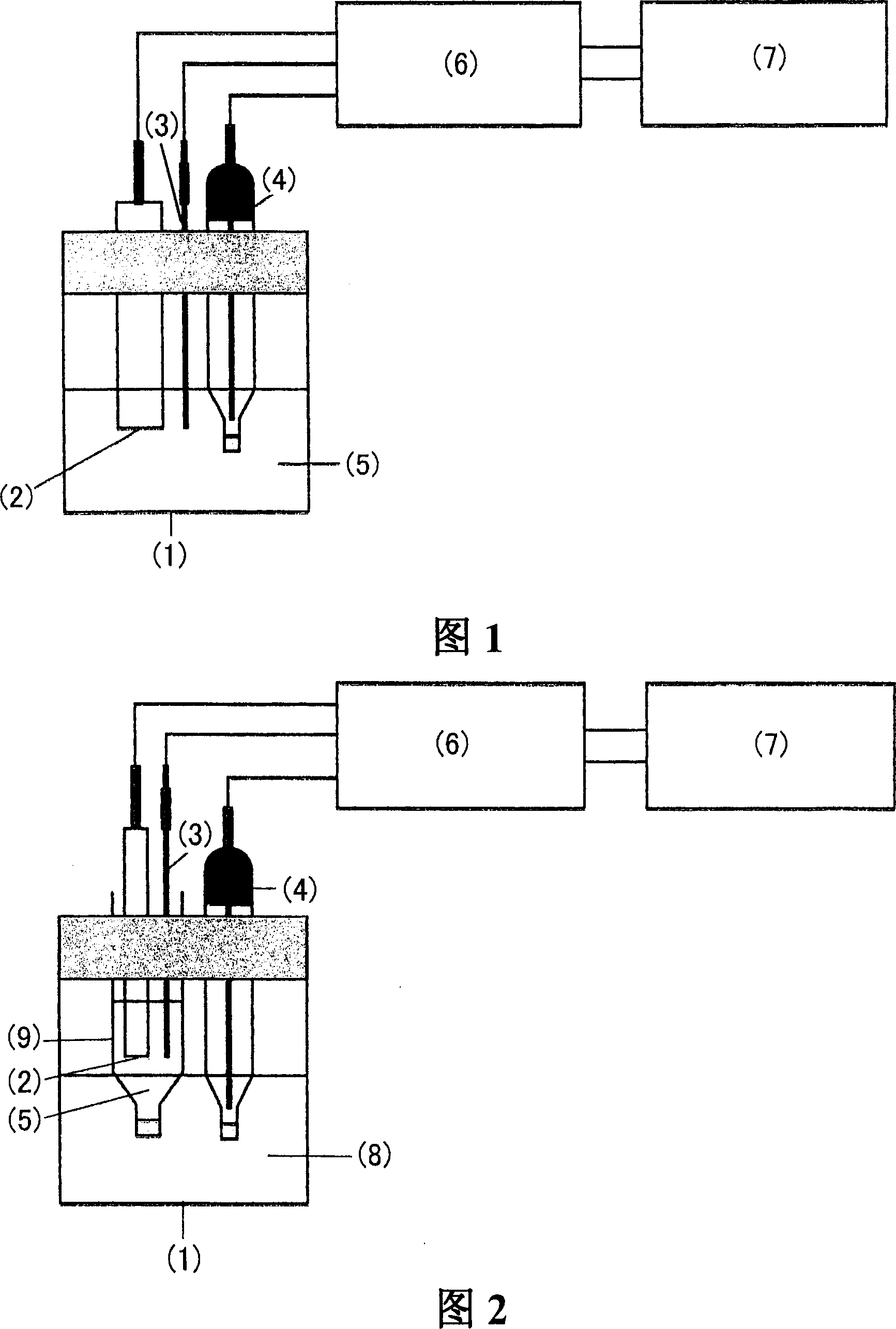
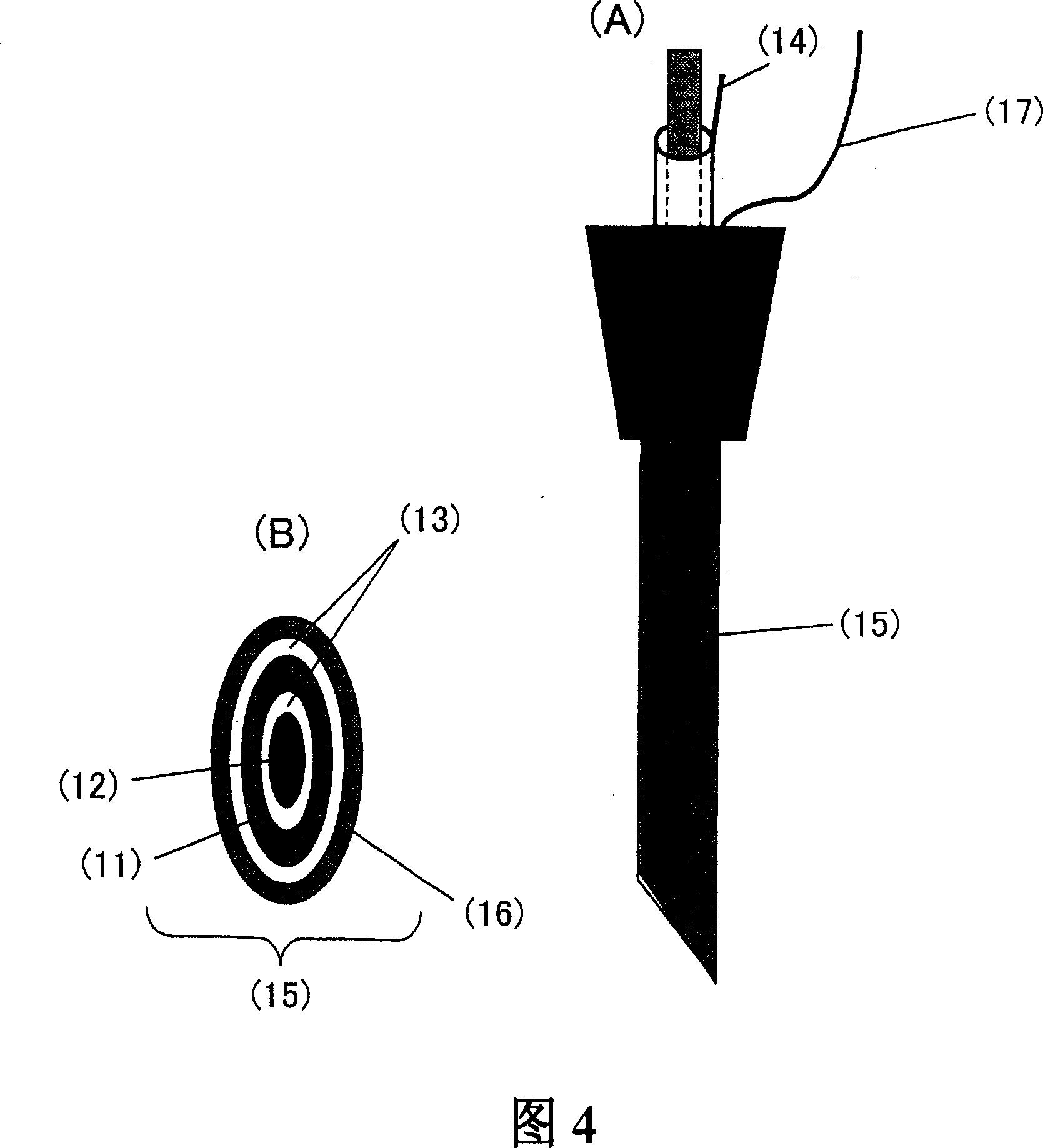
Electrode for superoxide anion and sensor using the same
A technology of peroxides and anions, used in material analysis, instruments, electrolytic coatings, etc. by electromagnetic means
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0069] Although the present invention will be described in more detail below with reference examples, examples, comparative examples, and test examples, the present invention is not limited thereto.
reference example 1
[0071] Synthesis of Metal Thiofuryl Porphyrin-Axial Ligand Coordination Compound (1):
[0072] Dissolve 0.036 g of 5,10,15,20-tetrakis(3-thiofuryl)porphyrinate iron(III) bromide and 200 μl of 1-methylimidazole in 1 ml of distilled dichloromethane at room temperature Stir for 5 hours. It was evaporated to dryness using an evaporating device to obtain [5,10,15,20-tetrakis(3-thiofuryl)porphyrin iron (III) bis(1-methylimidazolyl)] bromide (yield was 92%).
[0073] UV(CHCl 3 , nm, λmax): 419.
[0074] Since the absorption wavelength (λmax=424nm) is different from that of the coordination compound without imidazole, it was confirmed that imidazole was coordinated. In addition, in the magnetic circular dichroism spectrum, the polarization angles around 580 nm and 600 nm were reduced due to the coordination of imidazole, thereby confirming the hexacoordinate structure.
[0075] The UV (ultraviolet) spectrum of the raw material 5,10,15,20-tetrakis(3-thiofuryl)porphyrinate iron(III...
reference example 2
[0077] Synthesis of Metal Thiofuryl Porphyrin-Axial Ligand Coordination Compound (2):
[0078] Dissolve 0.041 g of 5,10,15,20-tetrakis(3-thiofuryl)porphyrinate iron(III) bromide and 0.08 g of 1-benzyl imidazole in 3 ml of distilled chloroform and stir at room temperature 5 hours. It was evaporated to dryness using an evaporator to obtain [5,10,15,20-tetrakis(3-thiofuryl)porphyrin iron(III)bis(1-benzylimidazolyl)]bromide (yield is 90%).
[0079] UV(CHCl 3 , nm, λmax): 418.
[0080] Since the absorption wavelength (λmax=424nm) is different from that of the coordination compound without imidazole, it was confirmed that imidazole was coordinated.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com