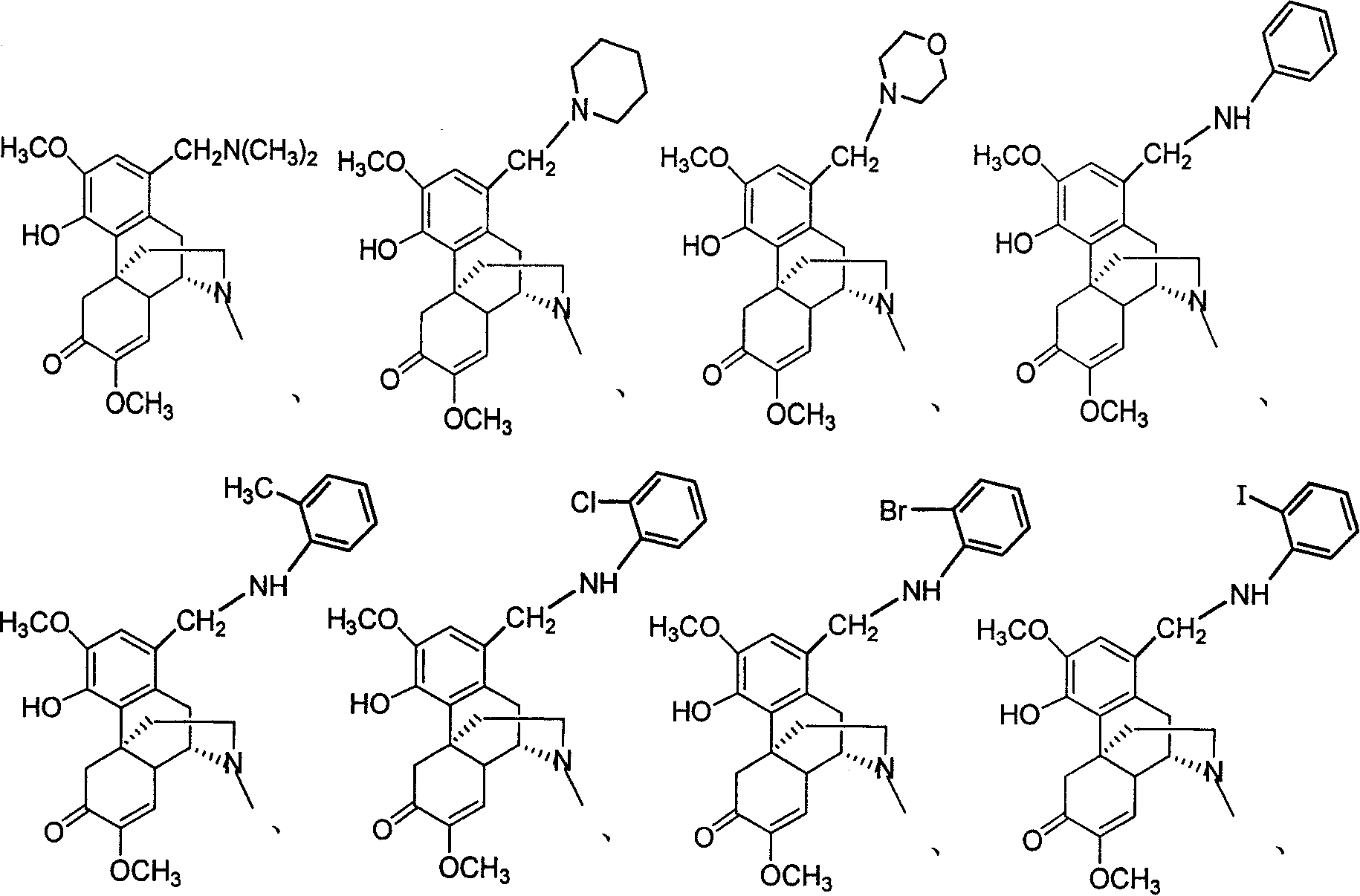
1-substituted amine methyl diversine derivative and its preparation method
A technology for aminomethyl sinomenine and derivatives, which is applied in the field of 1-substituted aminomethyl sinomenine derivatives and their preparation, can solve the problems of easy allergy and large dosage, and achieves high reaction yield and easy control. , mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] Method 1: Add 366mg (1mmol) of sinomenine hydrochloride and 2ml of absolute ethanol to the reaction flask, add hexahydropyridine (2mmol) and 0.153ml of formaldehyde aqueous solution (36%) (2mmo) under ice-bath stirring, at 0-20 Reaction at ℃ for 8-12h. At the end of the reaction, a small amount of distilled water and dichloromethane were added to extract 3 times, the organic layers were combined, and anhydrous MgSO 4 After drying, the dichloromethane was distilled off under reduced pressure and purified by silica gel column chromatography using dichloromethane:methanol (V:V=7:1) as the eluent to obtain (compound 2) 311 mg of a white solid with a yield of 73%.
[0030] Method 2: Add 366mg (1mmol) of sinomenine hydrochloride, hexahydropyridine (2mmol) and aqueous formaldehyde (0.153ml, 2mmol) (36%) in the reaction bottle, fix the mixture in the highest energy region of the ultrasonic cleaner at room temperature Ultrasound radiation for 10 to 20 minutes. At t...
Embodiment 2
[0037]
[0038] Method one: add sinomenine hydrochloride 366mg (1mmol) in reaction bottle, 2ml absolute ethanol, add 0.297ml dimethylamine aqueous solution (33%) (2mmol) and 0.153ml formaldehyde aqueous solution (36%) under stirring in ice bath ( 2mmol), react at 0-20°C for 8-12h. After the reaction, place it in the refrigerator overnight, filter it with suction, rinse with a small amount of ethanol, CH 2 Cl 2 -CH 3 COOCH 2 CH 3 After recrystallization, 305 mg of (compound 3) was obtained as a white solid, with a yield of 79%.
[0039] Method 2: Add 366mg (1mmol) of sinomenine hydrochloride, 0.297ml aqueous dimethylamine (33%) (2mmol) and 0.153ml aqueous formaldehyde (36%) (2mmol) in the reaction bottle, and fix the mixture in an ultrasonic cleaner The region with the highest energy is irradiated with ultrasound at room temperature for 10-20 minutes. After the reaction, place it in the refrigerator overnight, filter it with suction, rinse with a small amount of ethano...
Embodiment 3
[0046]
[0047] Add compound 3 (387mg, 1mmol) and 2mmol o-methoxyaniline to the reaction bottle, dissolve with an appropriate amount of 50% ethanol aqueous solution, and stir at room temperature for 8-12h. After the reaction, distill ethanol out under reduced pressure, add dichloromethane to extract three times , combined organic layers, anhydrous MgSO 4 After drying, the dichloromethane was distilled off under reduced pressure, and purified by silica gel column chromatography using dichloromethane:methanol (V:V=10:1) as eluent to obtain 232 mg of (compound 4) as a white solid, with a yield of 50%.
[0048] Mp: 121-122°C.
[0049] 1 H NMR (CDCl 3 , 400MHz) δ: 6.89(t, 1H, J=8.0Hz.), 6.79(d, 1H, J=8.0Hz.), 6.74(s, 1H), 6.70(t, 1H, J=8.0Hz.) , 6.61(d, 1H, J=8.0Hz.), 6.00(s, 1H), 5.45(d, 1H, J=1.6Hz.), 4.38(d, 1H, J=15.6Hz.), 4.10-4.19 (m, 2H), 3.80(s, 3H), 3.79(s, 3H), 3.50(s, 4H), 3.25(brs, 1H), 3.03-3.08(m, 2H), 2.58-2.68(m, 2H ), 2.47(d, 1H, J=15.6Hz.), 2.42(s, 3H), 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com