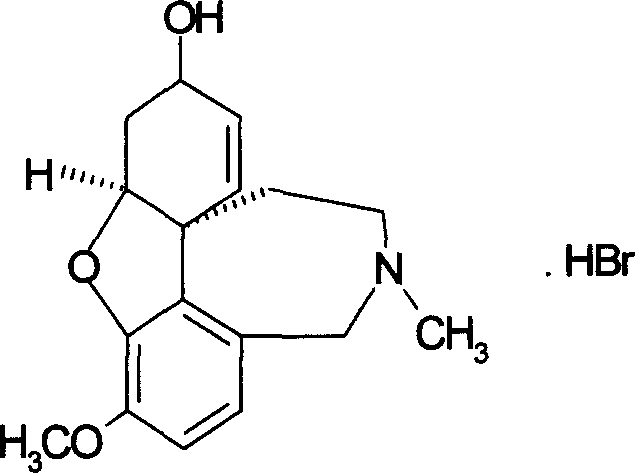
Orally disintegrated galantamine hydrobromide tablet and its prepn process
A technology of galantamine hydrobromide and hydrobromic acid is applied in the directions of pill delivery, nervous system diseases, active ingredients of heterocyclic compounds, etc., and can solve the problems of adverse effects of disease treatment, difficult swallowing of drugs, inconvenient use, and the like, Achieving stable blood drug concentration levels, improving compliance, and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0050] Example 1: An orally disintegrating tablet of immediate-release galantamine hydrobromide and its preparation method
[0051] The orally disintegrating tablet of galantamine hydrobromide is composed of main drug and auxiliary materials, and the ratio is: main drug 8.5%, auxiliary materials 91.5%. The proportion of each component is as follows:
[0052] Galantamine Hydrobromide 5.5g
[0053] Microcrystalline Cellulose 45.0g
[0054] Low-substituted hydroxypropyl cellulose 5.0g
[0055] Cross-linked polyvinylpyrrolidone 4.5g
[0056] Micronized silica gel 2.0g
[0057] Magnesium Stearate 2.0g
[0058] Stevioside 1.0g
[0059] Sweet orange solid essence 0.01g
[0060] A total of 1000 pieces were made
[0061] The preparation method of the galantamine hydrobromide disintegrating tablet of this example is as follows: sieve the galantamine hydrobromide and each auxiliary material through 80 sieves, and set aside. Weigh galanthamine hydrobromide and auxiliary materials a...
example 2
[0066] Example 2: A slow-release galantamine hydrobromide orally disintegrating tablet and its preparation method
[0067] Suspend 9 g of 200 mesh sulfonic acid cation exchange resin (Amberlite IRP 64, Rohm&Hass Company) in 150 ml of purified water, add 5.0 g of galantamine hydrobromide, heat and stir at 60°C for 4 hours, filter, and dry at 80°C to obtain Galantamine hydrobromide-sulfonic acid type cation exchange resin drug adsorbate. The drug adsorbate was added to 150ml of 0.5% ethylcellulose cyclohexane solution, heated and stirred, refluxed for 1 hour, cooled to 10°C, filtered, and dried at 60°C for 12 hours to obtain the coated drug adsorbate.
[0068] Sustained-release galantamine hydrobromide orally disintegrating tablets were prepared according to the following formula:
[0069] Coated drug adsorbate 14.75g
[0070] Mannitol 25.0g
[0071] Microcrystalline Cellulose 20.0g
[0072] Cross-linked polyvinylpyrrolidone 4.0g
[0073] Low-substituted hydroxypropyl cellu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fineness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com