Terrein compound having melanin biosynthesis inhibitors and its preparation
A technology of biosynthesis and terreus, applied in biochemical equipment and methods, methods based on microorganisms, organic active ingredients, etc., can solve problems such as death, deterioration of chromaffin cells, damage to original cell functions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] preparation of terreinone compound of the present invention
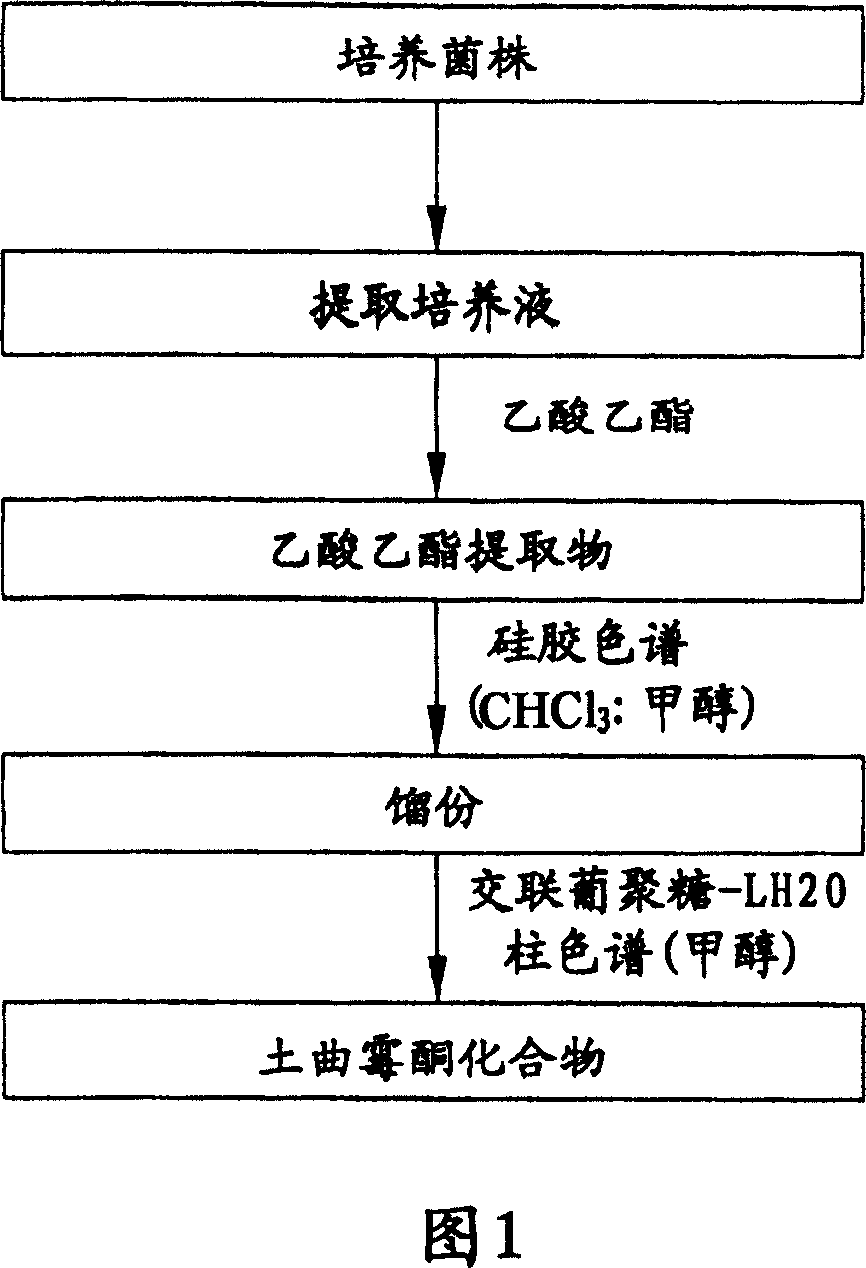
[0049] The melanin biosynthesis inhibitor of the present invention can be isolated from the broth medium of Penicillium sp strain isolated from soil.
[0050] Specifically, in a 500 ml Erlenmeyer flask, Penicillium sp F020135 (Accession No.: KCTC 26245) (a fungal strain isolated from soil) was placed on 100 ml of yeast-malt extract (YM) medium at 140 rpm Cultured at 28°C for 8 days. Cells and culture medium were separated from each other using filter paper, and cells were placed in an equal volume of acetone overnight. Cells were removed by filtration with filter paper, and concentrated under reduced pressure to obtain an acetone extract. The acetone extract and the culture medium were placed in an equal amount of ethyl acetate for further extraction. The mixture was concentrated under reduced pressure to obtain ethyl acetate extract.
[0051] Fractions were obtained from the prepared ethyl acetate extra...
preparation Embodiment 1
[0060] Preparation of a cream containing the terrein compound of the present invention
[0061] Put stearic acid, cetostearyl alcohol, caprylic / capric triglyceride, mineral oil and butylene glycol into a beaker placed in a water bath, and heat to 75°C to form an oil phase. Mix the terreinone compound prepared in the above-mentioned Example 1, water, glycerin, Tween 60, Tween 80 and potassium hydroxide to form a water phase, and add the above-mentioned oil phase to the water phase. The reaction solution was stirred at 1200-1500 rpm for 10-20 minutes and then cooled. The solution was left at room temperature for 1-2 days. The contents of the substances contained in the cream are shown in Table 1 below (the total weight is 100 g).
[0062] substance
experiment Embodiment 1
[0063] Cytotoxicity test of the compound of the present invention
[0064] To study the cytotoxicity of the terreinone compounds of the present invention, Mel-Ab cells were treated with the terreinone compounds.
[0065] Specifically, at 5% CO 2 , Under the condition of 37°C, Mel-Ab melanocytes were cultivated with DMEM medium supplemented with 10% fetal bovine serum (FBS), 100nM phorbol 12-myristate 13-acetate (phobol 12-myristate 13-acetate), 1 nM cholera toxin, 50 μg / ml streptomycin and 50 U / ml penicillin.
[0066] The Mel-Ab melanocytes cultured above were treated with terreinone compound at different concentrations (0, 10, 25, 50, 75, 100 μM) for 24 hours, and then further cultured. The medium is then removed. Cells were stained with 0.5 ml 0.1% crystal violet and checked for Mel-Ab melanocytes. Crystal violet was removed by washing several times. Use 1.0 ml of 95% ethanol to extract the remaining crystal violet in the cells. Measure the OD of the extracted crystal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com