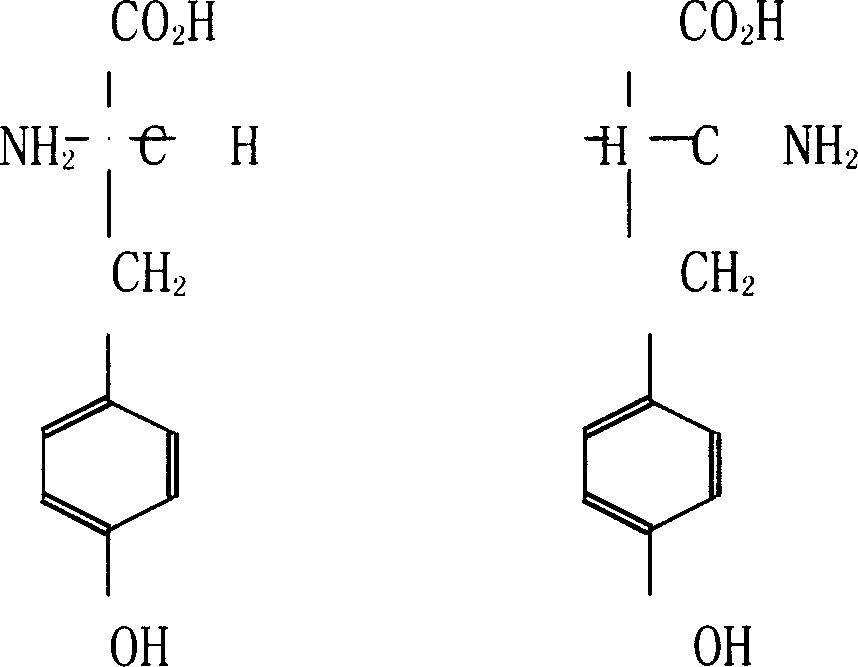
Method for preparing D-tyrosine by enzyme method
A technology for enzymatic preparation of tyrosine, which is applied in fermentation and other directions, can solve rare problems and achieve the effects of strong anti-inhibition, reduced production costs, and strong stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Step 1 Preparation of N-Acetyl-DL-Tyrosine
[0025] Dissolve 36.2g of DL-tyrosine (0.2mol) and 24.0g of acetic acid (0.4mol) in 200ml of deionized water, add 24.0g of acetic anhydride (0.2mol), stir and react at 40-60°C for 3-6 hours, distill Unreacted acetic acid was recovered, cooled to room temperature and then cooled in an ice-water bath for 1 hour, then filtered, centrifuged, washed, and dried to obtain 42.6 g of product with a yield of 95.5%. The obtained N-acetyl-DL-tyrosine is used for later use.
[0026] Step 2 Purification and immobilization of D-acylated hydrolase
[0027] The commercially available D-acylase hydrolase cells are added to a dissolving tank (PH7.8), partially purified with a diethylaminoethyl chromatographic column, immobilized with an enzyme column, and stored at low temperature for future use.
[0028] After testing, each gram of wet bacteria can produce 2245 activity units of D-acylase and 852 activity units of L-acylase. The immobilized ...
Embodiment 2
[0032] Step 1 Preparation of N-Acetyl-DL-Tyrosine
[0033] Dissolve 54.3g of DL-tyrosine (0.3mol) and 36.0g of acetic acid (0.6mol) in 200ml of deionized water, add 24.0g of acetic anhydride (0.2mol), stir and react at 50-65°C for 4-6 hours, distill Recover unreacted acetic acid, cool to room temperature and then cool in an ice-water bath for 45 minutes, then filter, centrifuge, wash, and dry to obtain 64.2 g of product with a yield of 96.0%. The obtained N-acetyl-DL-tyrosine is ready for use.
[0034] Step 2D - Purification and immobilization of the acylase hydrolase
[0035] The enzyme column prepared in step 2 in Example 1 was used.
[0036] Step 3D-Acylation hydrolase splits gyro-tyrosine to prepare D-tyrosine
[0037] 64.2 g of N-acetyl-DL-tyrosine prepared in the above step 1 was dissolved in 300 ml of deionized water, and the pH value was adjusted to 7.5 with ammonia water. The reaction solution was passed through the immobilized enzyme column at 50°C, and then elut...
Embodiment 3
[0039]Step 1 Preparation of N-Acetyl-DL-Tyrosine
[0040] Dissolve 72.4g of DL-tyrosine (0.4mol) and 42.0g of acetic acid (0.7mol) in 350ml of deionized water, add 24.0g of acetic anhydride (0.2mol), stir and react at 50-60°C for 4-5 hours, distill Unreacted acetic acid was recovered, cooled to room temperature and then cooled in an ice-water bath for 1.5 hours, then filtered, centrifuged, washed, and dried to obtain 84.4 g of product with a yield of 94.6%. The obtained N-acetyl-DL-tyrosine is used for later use.
[0041] Purification and immobilization of step 2D-type aminoacyl hydrolase
[0042] The enzyme column prepared in step 2 in Example 1 was used.
[0043] Step 3D-Acylation hydrolase splits gyro-tyrosine to prepare D-tyrosine
[0044] Dissolve 84.4 g of N-acetyl-DL-tyrosine prepared in step 1 above in 500 ml of deionized water, and adjust the pH value to 7.5 with sodium hydroxide solution. The reaction solution was passed through the immobilized enzyme column at 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com