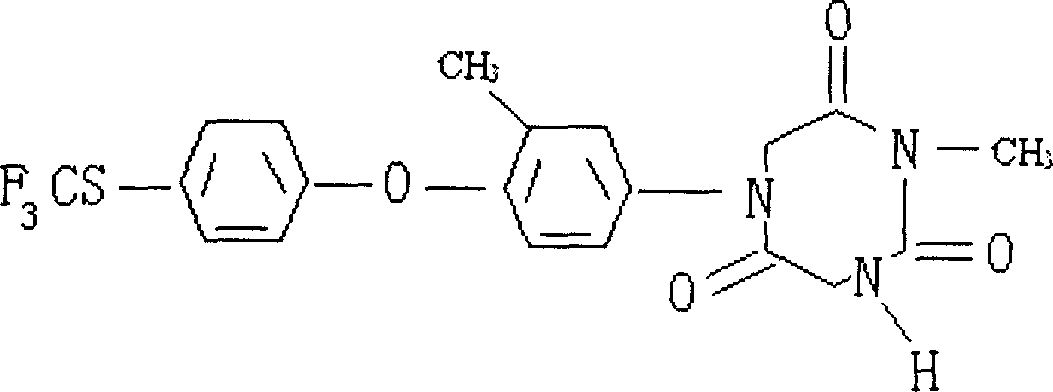
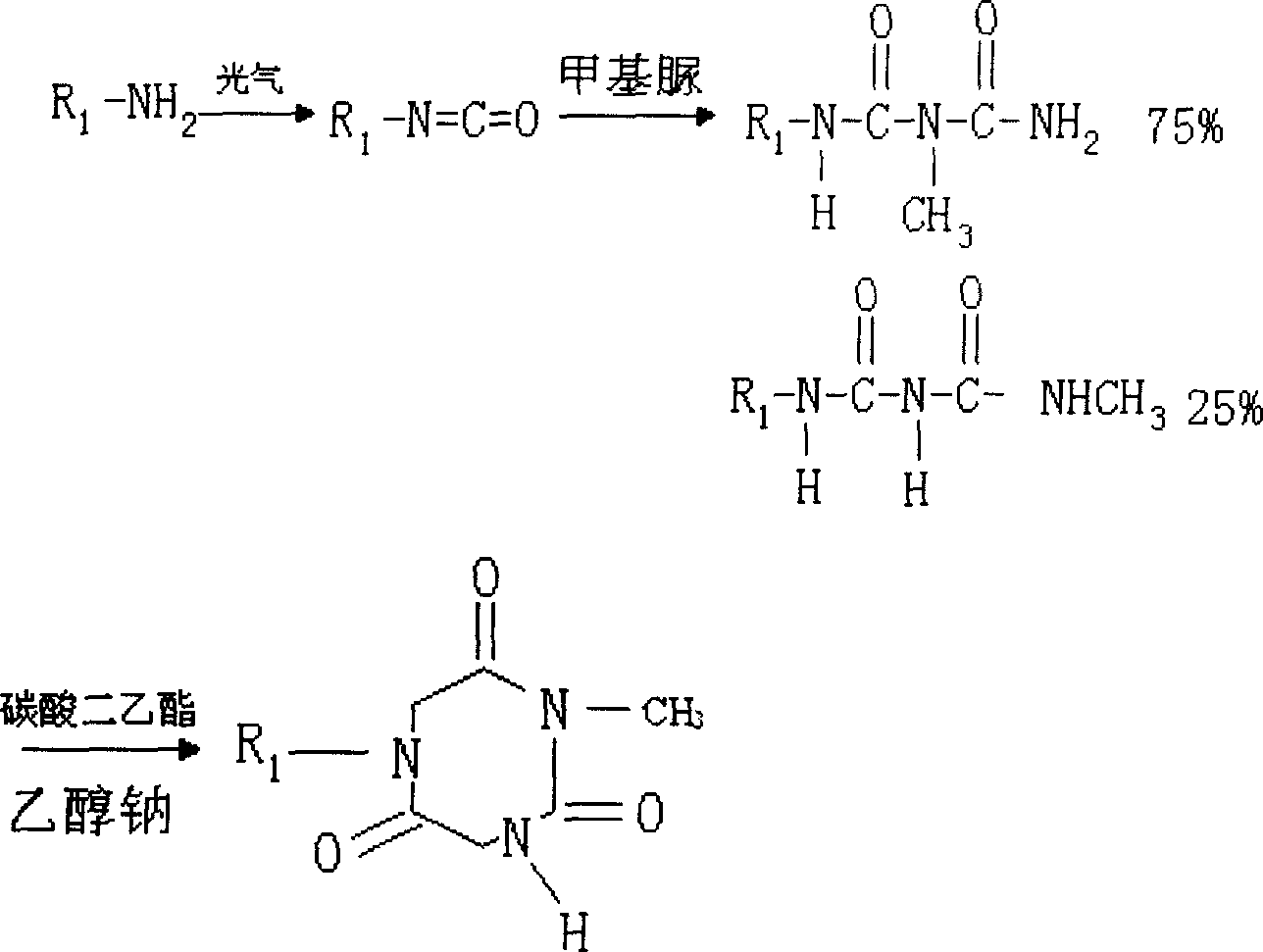
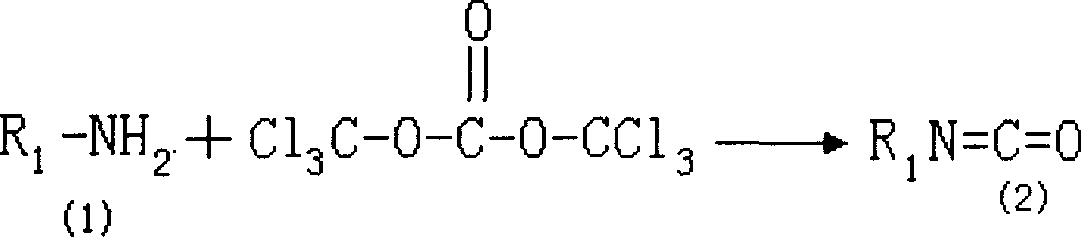Production of tolyl-triazone
A technology of toltriazinone and a new method is applied in the new field of preparation of toltriazinone, and can solve the problems of large injury to production and operation workers, strong phosgene toxicity, inability to purify and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0026] ① Synthesis of 3-methyl-4-(4-trifluoromethylthio)phenoxybenzene isocyanate (2):
[0027] Dissolve 299g of 3-methyl-4-(4-trifluoromethylthio)phenoxyaniline (1) in 5000ml of anhydrous toluene, feed hydrogen chloride to saturation, and add 200g of hexachlorohydrin dropwise under zero-degree cooling Add the solution of dimethyl carbonate / 2000ml toluene in about 4 hours, then slowly raise the temperature to reflux and keep it warm for 4 hours, remove the toluene under reduced pressure, and distill to receive 160℃~166℃ / 1mmHg fraction to obtain 3- Methyl-4-(4-trifluoromethylthio)phenoxybenzene isocyanate (2) 305g, yield 93%;
[0028] ② 1(N)-acetyl-2(N)-methyl-3(N)-[3-methyl-4-(4-trifluoromethylthio)phenoxy]phenyltriurea (3) preparation:
[0029] Dissolve 162.5 g of 3-methyl-4-(4-trifluoromethylthio)phenoxybenzene isocyanate (2) in 500 ml of chloroform, add 70 g of N-acetyl-N'-methylurea, Heating and reflux reaction, followed by TLC until the disappearance of the raw materia...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com