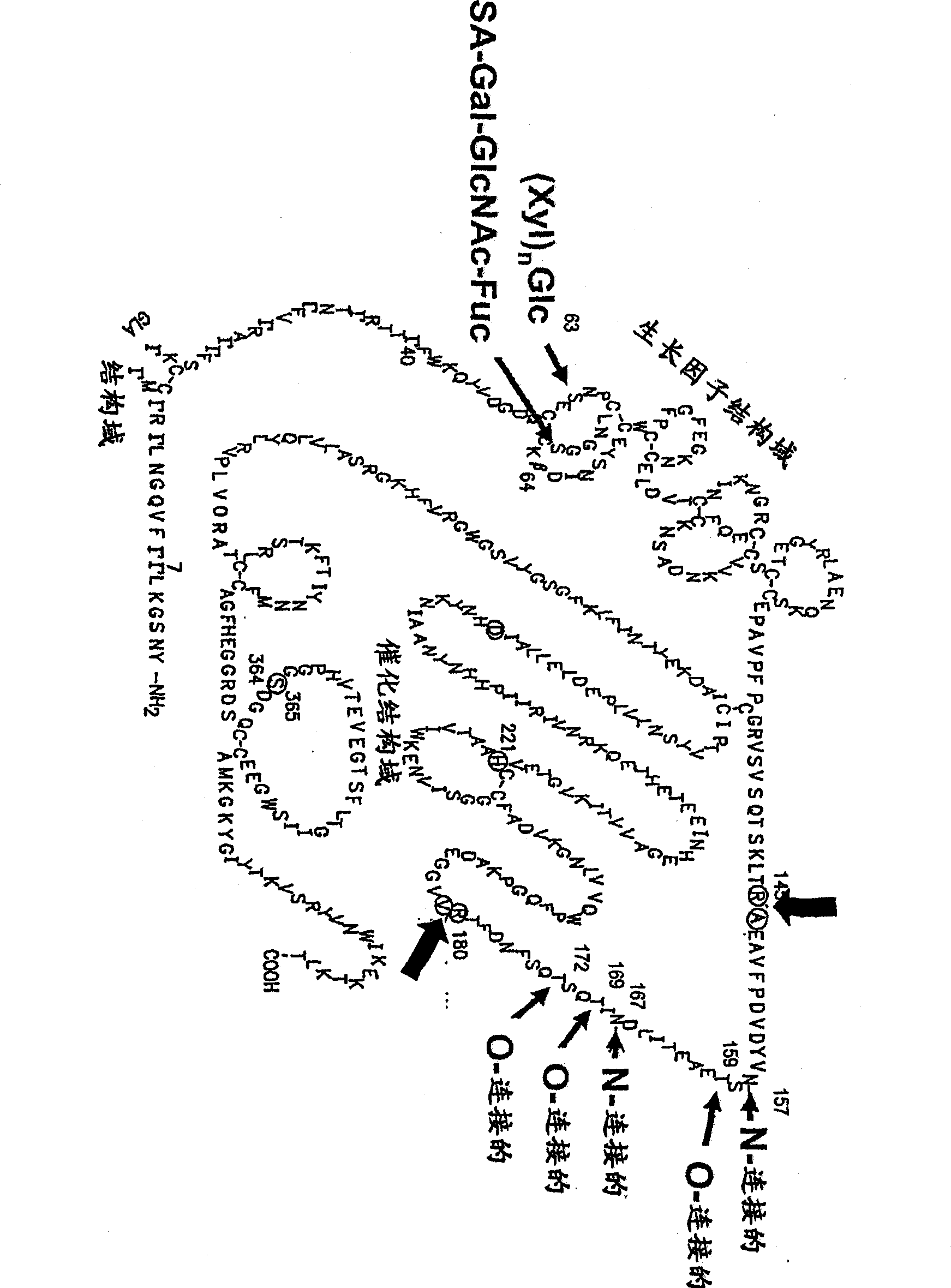
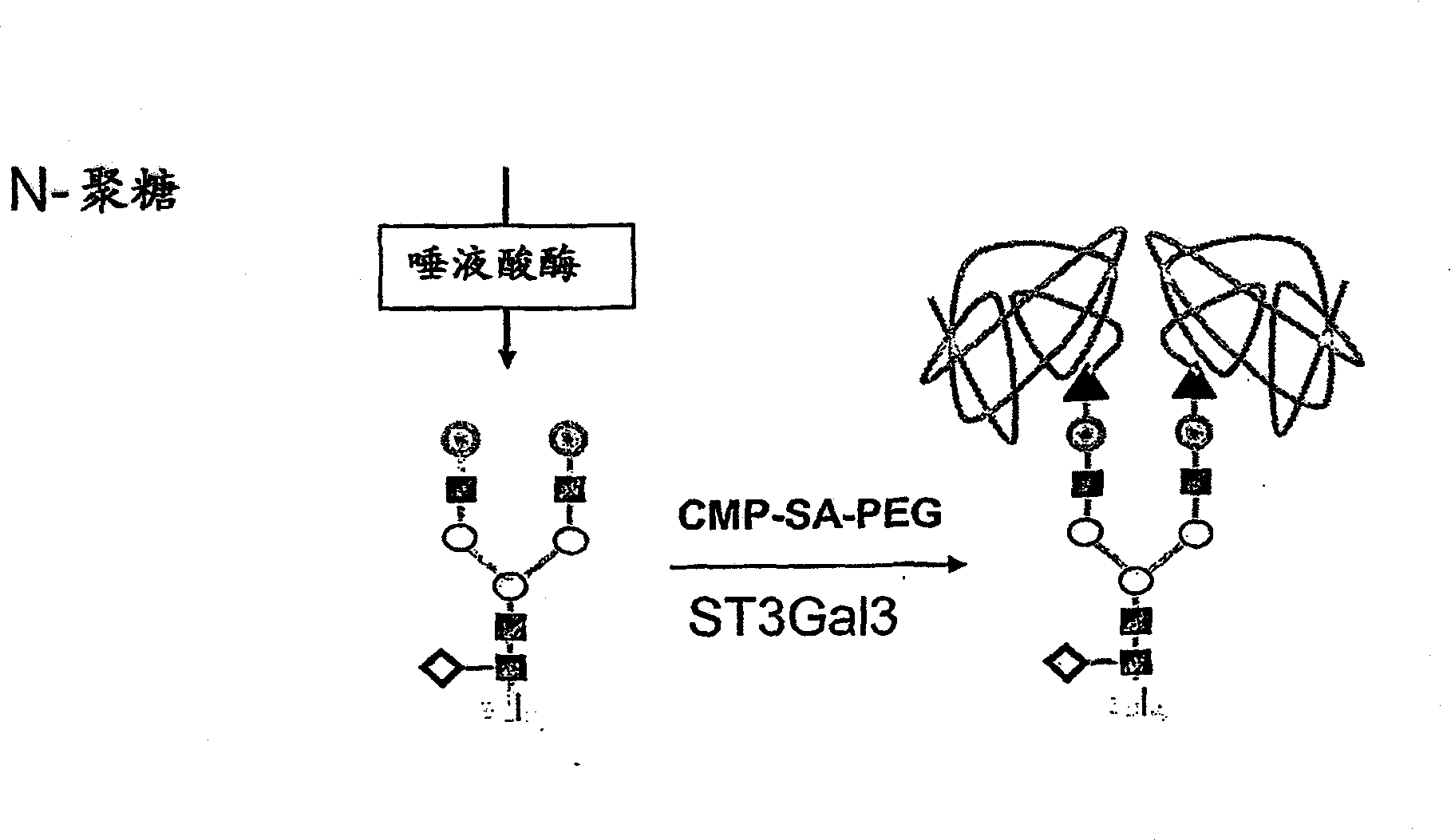
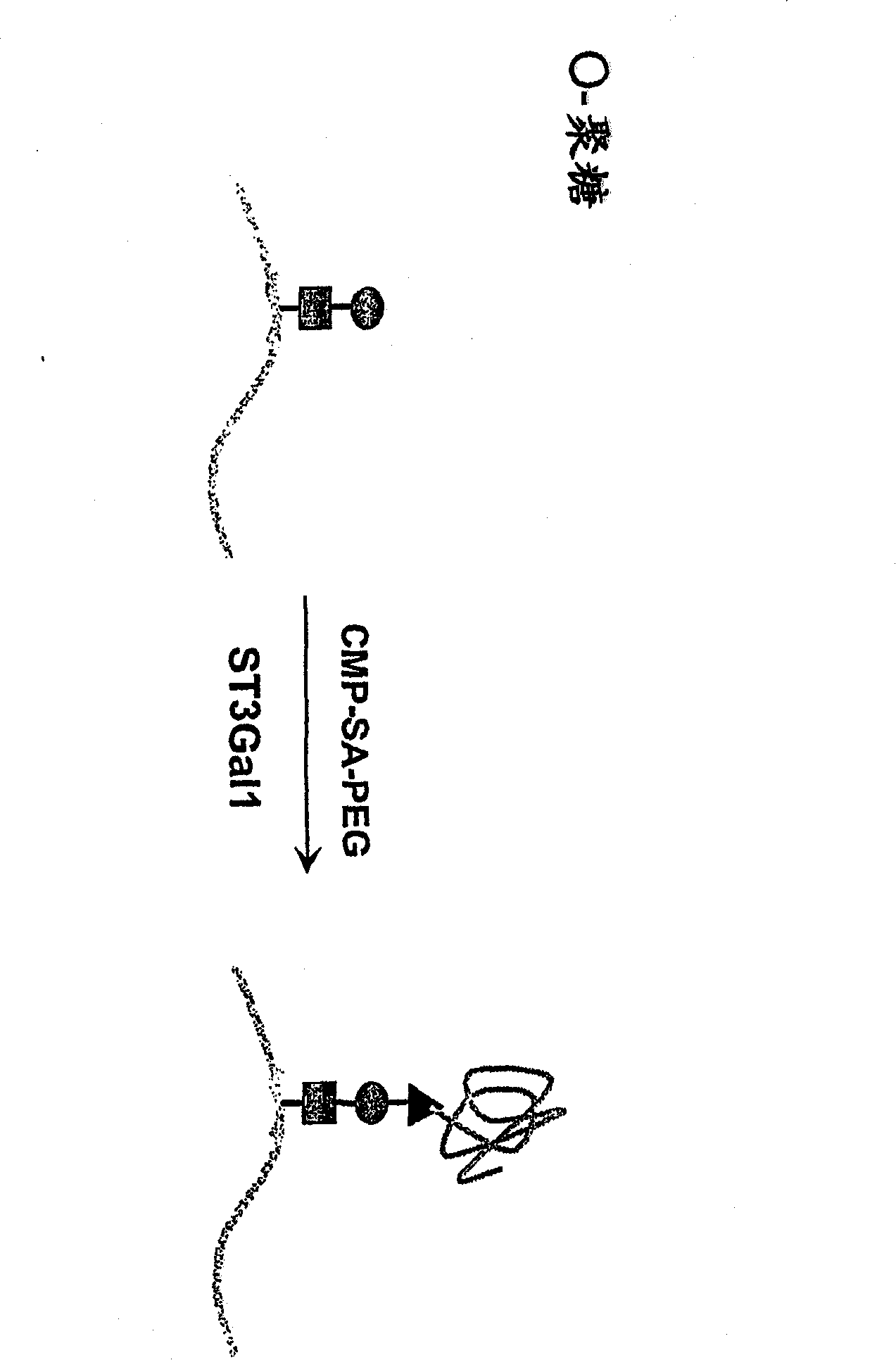
Glycopegylated factor IX
A polyethylene glycol, factor technology, applied in the production of these factor IX peptides, the field of manufacturing PEGylated factor IX comprising the above-mentioned parts, can solve the problems of lack of final product homogeneity, reduction of peptide biology or enzymatic activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0449] Preparation of UDP-GalNAc-6'-CHO
[0450] Dissolve UDP-GalNAc (200 mg, 0.30 mmole) in 1 mM CuSO 4 solution (20mL) and 25mM NaH 2 PO 4 solution (pH 6.0, 20 mL). Then galactose oxidase (240 U, 240 μL) and catalase (13000 U, 130 μL) were added, and the reaction system was assembled with an oxygen-filled balloon and stirred at room temperature for seven days. The reaction mixture was then filtered (centrifuge tube, MWCO 5K) and the filtrate (-40 mL) was stored at 4°C until use. TLC (silica; ethanol / water (7 / 2); R f =0.77; visualization of anisaldehyde staining).
Embodiment 2
[0452] Preparation of UDP-GaINAc-6'-NH 2
[0453] To the above UDP-GalNAc-6'-CHO solution (2 mL or ~20 mg) was added ammonium acetate (15 mg, 0.194 mmole) and NaBH at 0 °C 3 CN (1M in THF; 0.17 mL, 0.17 mmole) and incubated overnight at room temperature. The reaction was filtered through an aqueous G-10 cartridge and the product was collected. Appropriate fractions were lyophilized and stored frozen. TLC (silica; ethanol / water (7 / 2); R f =0.72; developed by ninhydrin staining).
Embodiment 3
[0455] Preparation of UDP-Ga I NAc-6-NHCO(CH 2 ) 2 -O-PEG-OMe(1KDa)
[0456] Galactosylamino-1-phosphate-2-NHCO (CH 2 ) 2 -O-PEG-OMe (1 KDa) (58 mg, 0.045 mmole) was dissolved in DMF (6 mL) and pyridine (1.2 mL). Then UMP-morpholidate (60 mg, 0.15 mmole) was added and the resulting mixture was stirred at 70°C for 48 hours. The solvent was removed by bubbling nitrogen through the reaction mixture and the residue was purified by reverse phase chromatography (C-18 silica, step gradient between 10 and 80%, methanol / water). The desired fractions were collected and dried under reduced pressure to obtain 50 mg (70%) of a white solid. TLC (silica, propanol / H 2 O / NH 4 OH, (30 / 20 / 2), Rf = 0.54). MS (MALDI): 1485, 1529, 1618, 1706 observed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com