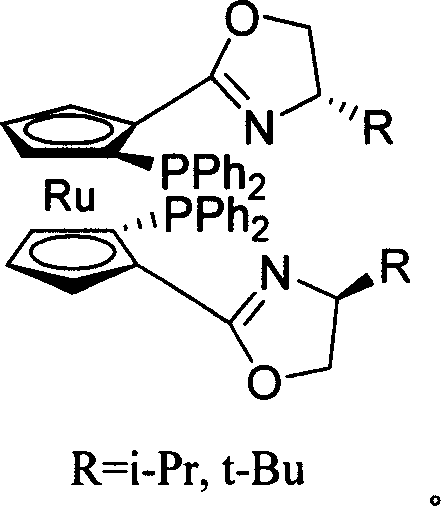
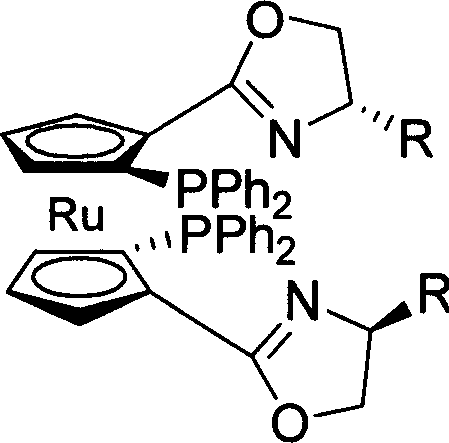
C2-symmetrical chirality bis ruthenium ligand and its synthesis method
A synthesis method and ruthenocene technology, which are applied in the field of C2-symmetric chiral ruthenocene ligands and their synthesis, can solve the problems that the same or similar literature reports have not been found, and achieve high reactivity and stereoselectivity, The effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Preparation of 1,3-cyclocene
[0030] Take 50mL of bicyclocene in a 150mL one-necked flask, under N 2 Raise the temperature to 180-200°C under protection, let the steam pass through the rectification column, collect the fraction at 40-42°C, and obtain a colorless transparent liquid, which is directly used in the next reaction.
[0031] 2. Preparation of ruthenocene
[0032] RuCl 3 *nH 2 O (5.26g, 20mmol) was dissolved in absolute ethanol (250mL), cyclocene (25mL) was added dropwise at room temperature, and then Zn powder was added in batches. The resulting gray suspension was stirred for 2 h, filtered, and the filter cake was washed with a large amount of toluene. The toluene phase was decolorized with activated carbon, dried over anhydrous magnesium sulfate, and the solvent was recovered under reduced pressure to obtain 4.3 g of light green crystals, y=85.3%.
[0033] 1 H NMR (400MHz, CDCl 3 ): δ4.55(s, 10H).
[0034] 3. Preparation of 1,1'-dicarboxyruthenoc...
Embodiment 2
[0045] 1. Preparation of 1,3-cyclocene
[0046] Take 50mL of bicyclocene in a 150mL one-necked flask, under N 2 Raise the temperature to 180-200°C under protection, let the steam pass through the rectification column, collect the fraction at 40-42°C, and obtain a colorless transparent liquid, which is directly used in the next reaction.
[0047] 2. Preparation of ruthenocene
[0048] RuCl 3 *nH 2 O (5.26g, 20mmol) was dissolved in absolute ethanol (250mL), cyclocene (25mL) was added dropwise at room temperature, and then Zn powder was added in batches. The resulting gray suspension was stirred for 2 h, filtered, and the filter cake was washed with a large amount of toluene. The toluene phase was decolorized with activated carbon, dried over anhydrous magnesium sulfate, and the solvent was recovered under reduced pressure to obtain 4.3 g of light green crystals, y=85.3%.
[0049] 1 H NMR (400MHz, CDCl 3 ): δ4.55(s, 10H).
[0050] 3. Preparation of 1,1'-dicarboxyruthenoc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com