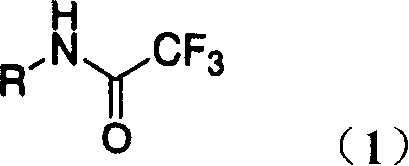
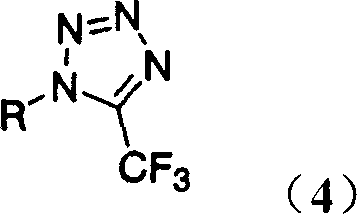
Process for production of 1-aryl-5-(trifluoromethyl)-1h- tetrazoles
A kind of aryl, trifluoroimidoacetyl technology, applied in directions such as organic chemistry, can solve problems such as difficult to remove, not favorable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] N-phenyl-2,2,2-trifluoroiminoacetyl chloride
[0062]
[0063] 7g (37.0mmol) 2,2,2-trifluoro-N-phenylacetamide, 19.84g (74.0mmol) diphenyl chlorophosphate, 7.44g (74.0mmol) triethylamine and 28ml acetonitrile were added to 100ml React in the flask for 15 hours while refluxing (82°C). After the reaction, the reaction solution was cooled to room temperature, 28 ml of ethyl acetate was added thereto, and then the precipitate was removed by filtration. The filtrate was evaporated to remove the solvent, and the obtained crude product was purified by column chromatography (silica gel, ethyl acetate: hexane = 3: 7) to obtain 6.24 g of N-phenyl-2,2,2-trifluoroimino Yellow liquid of acetyl chloride (yield: 81.2%).
[0064] IR (pure, cm -1 ): 1697, 1489, 1286, 1223, 1196, 1161, 947, 766, 725, 691
[0065] 1 H-NMR (CDCl 3 ): δ7.41-7.24 (m, 3H), 7.08-7.05 (m, 2H)
[0066] 13 C-NMR (CDCl 3 ): δ143.47, 131.94 (q, J=42.8Hz), 129.12, 127.40, 120.63, 116.86 (q, J=275.8Hz)
Embodiment 2
[0068] 1-Phenyl-5-(trifluoromethyl)-1H-tetrazole
[0069]
[0070] 5g (24.1mmol) of N-phenyl-2,2,2-trifluoroiminoacetyl chloride obtained in Example 1, 2.83g (43.4mmol) of sodium azide, 1.66g (12.1mmol) of hydrochloric acid tris Ethylamine and 40ml of toluene were added into a 100ml flask and reacted at 80°C for 16.5 hours. After the reaction, the reaction solution was cooled to room temperature and washed with water (30ml×3). The organic phase was dried over anhydrous magnesium sulfate for 1 hour, filtered and the solvent was evaporated. The obtained crude product was purified by column chromatography (silica gel, ethyl acetate: hexane = 3: 7) to obtain 4.81 g of 1-phenyl-5-(trifluoromethyl)-1H-tetrazole as a pale yellow oil (Yield: 93.2%).
[0071] IR (pure, cm -1 ): 3071, 1531, 1499, 1312, 1207, 1167, 1013, 766, 691
[0072] 1 H-NMR (CDCl 3 ): δ7.60-7.54(m, 3H), 7.38(d, J=8.7Hz, 2H), 7.06(d, J=8.7Hz, 2H), 3.89(s, 3H)
[0073] 13 C-NMR (CDCl 3 ): δ145.90 (q, J=42....
Embodiment 3
[0079] N-(4-methylphenyl)-2,2,2-trifluoroiminoacetyl chloride
[0080]
[0081] 7g (34.5mmol) 2,2,2-trifluoro-N-(4-methylphenyl) acetamide, 18.49g (68.9mmol) diphenyl chlorophosphate, 6.97g (68.9mmol) triethylamine and 35ml of acetonitrile was added into a 100ml flask to react for 18 hours while refluxing (82°C). After the reaction, the reaction solution was cooled to room temperature, 25 ml of ethyl acetate was added, and the precipitate was removed by filtration. The filtrate was evaporated to remove the solvent, and the obtained crude product was purified by column chromatography (silica gel, ethyl acetate: hexane = 3: 7) to obtain 6.77 g of N-(4-methylphenyl)-2,2,2 - Yellow liquid of trifluoroacetyl chloride (yield: 88.6%).
[0082] IR (pure, cm -1 ): 1684, 1506, 1286, 1223, 1196, 1159, 949, 934, 820
[0083] 1 H-NMR (CDCl 3 ): δ7.26-7.22(m, 2H), 7.10-7.04(m, 2H), 2.39(s, 3H)
[0084] 13 C-NMR (CDCl 3 ): δ140.61, 137.85, 130.55 (q, J=42.8Hz), 129.69, 121.23, 11...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com