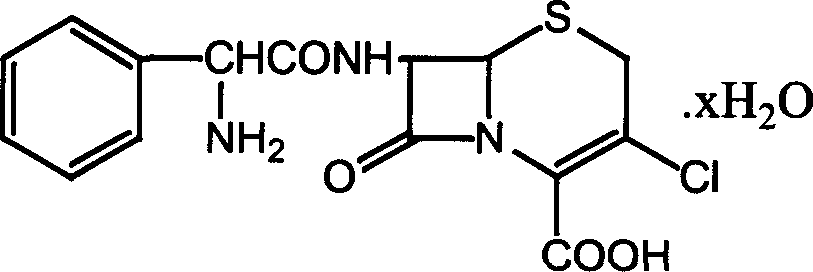
Method for recovery of cefaclor
A technology of cefaclor and recovery method, which is applied in the field of recovery of cefaclor, can solve problems such as not involving transformation, achieve the effect of reducing losses, simple recovery method, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In the 10000ml reaction bottle, add cefaclor content and be 6000ml of cefaclor aqueous solution of 14.78g / l, under stirring, add 80g pulverized 1-naphthol (particle size is less than 100 μm) at room temperature, adjust the solution so that the pH of the solution is 4-5, stir at room temperature for 1-2 hours, then cool down to 0-5°C, stir and react for 2-3 hours, filter, wash with appropriate amount of water and acetone, and vacuum-dry at 30-35°C to obtain light yellow cefaclor complex 137.1g (80.87% dry content). The residual cefaclor in the aqueous solution of cefaclor was 1.02 g / l, and the recovery rate was about 95%.
[0034] Add 100 g of the prepared cefaclor complex in portions to a mixed solution of 100 ml of water and 500 ml of N, N-dimethylformamide or N, N-dimethylacetamide, keep the temperature at 10-20 ° C, and use Adjust the pH to 1.5-2.0 with concentrated hydrochloric acid to dissolve, filter, wash with 50ml N,N-dimethylformamide or N,N-dimethylacetamide,...
Embodiment 2
[0037] In a 10000ml reaction bottle, add 6000ml of cefaclor aqueous solution with cefaclor content of 14.78g / l, under stirring, add 85g of pulverized 2-naphthol (particle size is less than 100μm) at room temperature, adjust the pH to 4~5, and stir at room temperature 1 to 2 hours, then lower the temperature to 0 to 5°C, stir and react for 2 to 3 hours, filter, wash with appropriate amount of water and acetone, and dry in vacuum at 30 to 35°C to obtain 121.5g of light yellow cefaclor complex (dried content was 80.46%). The cefaclor aqueous solution contained 2.35 g / l of cefaclor, and the recovery rate was about 84%.
[0038]Add 100 g of the prepared cefaclor complex into 100 ml of water and 500 ml of N,N-dimethylformyl or N,N-dimethylacetamide mixed solution in portions, keep the temperature at 10-20°C, and use concentrated Adjust the pH to 1.5-2.0 with hydrochloric acid to dissolve, filter, wash with 50ml N,N-dimethylformamide or N,N-dimethylacetamide, combine the filtrate an...
Embodiment 3
[0041] In a 1000ml reaction flask, add 200ml of cefaclor aqueous solution with a cefaclor content of 14.78g / l, cool down to 0-10°C under stirring, add 600ml of N,N-dimethylformamide or N,N-dimethylformamide Methyl acetamide, adjust the pH to 6-7, keep at 20-28°C, stir for 1 hour, cool down to 5-10°C, stir for 1-2 hours, filter, wash with an appropriate amount of ethyl acetate, and vacuum-dry at 30-35°C to obtain white Cefaclor N, N-dimethylformamide complex or cefaclor N, N-dimethylacetamide complex is about 3.51 g (74.17% on a dry basis). The residual cefaclor in the mother liquor was 1.47g / l, and the recovery rate was about 87%.
[0042] Then the obtained cefaclor N, N-dimethylformamide complex or cefaclor N, N-dimethylacetamide complex is dissolved in water under the condition of adding acid, and the pH value is adjusted with alkali After making it crystallized, the solid and liquid are separated, and then washed with a washing solvent to obtain crystallized cefaclor hydra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com