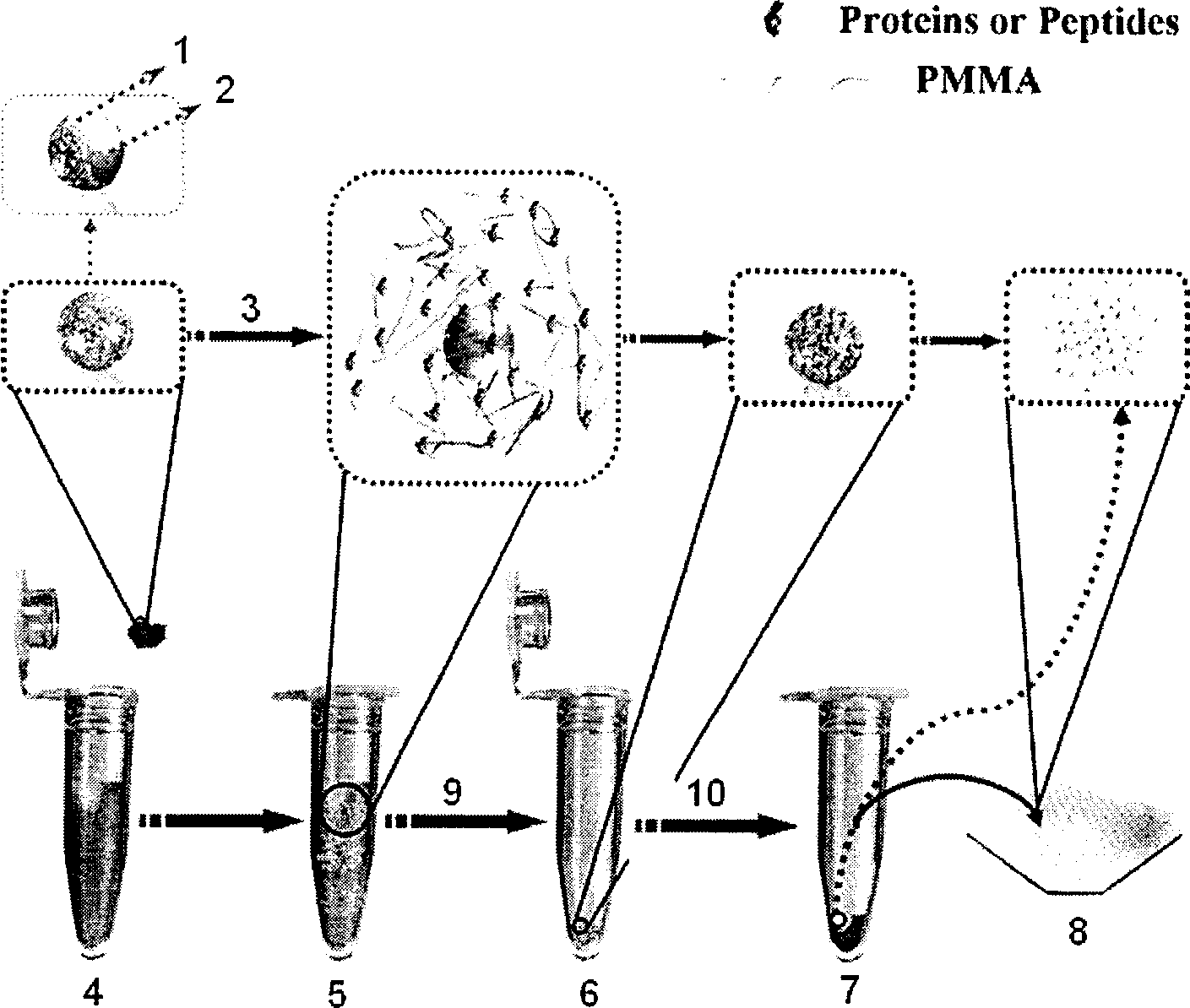
Method for fast enriching trace polypeptide and protein and realizing identification
A protein and enrichment technology, applied in the field of biochemical analysis, can solve the problems of easily polluting the ion optical channel of mass spectrometry, affecting signal stability and reproducibility, and long enrichment time, so as to ensure reproducibility and stability, avoid Sample loss, good mass spectrum reproducibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] CaCO 3 -Study on the adsorption behavior of PMMA materials to peptides
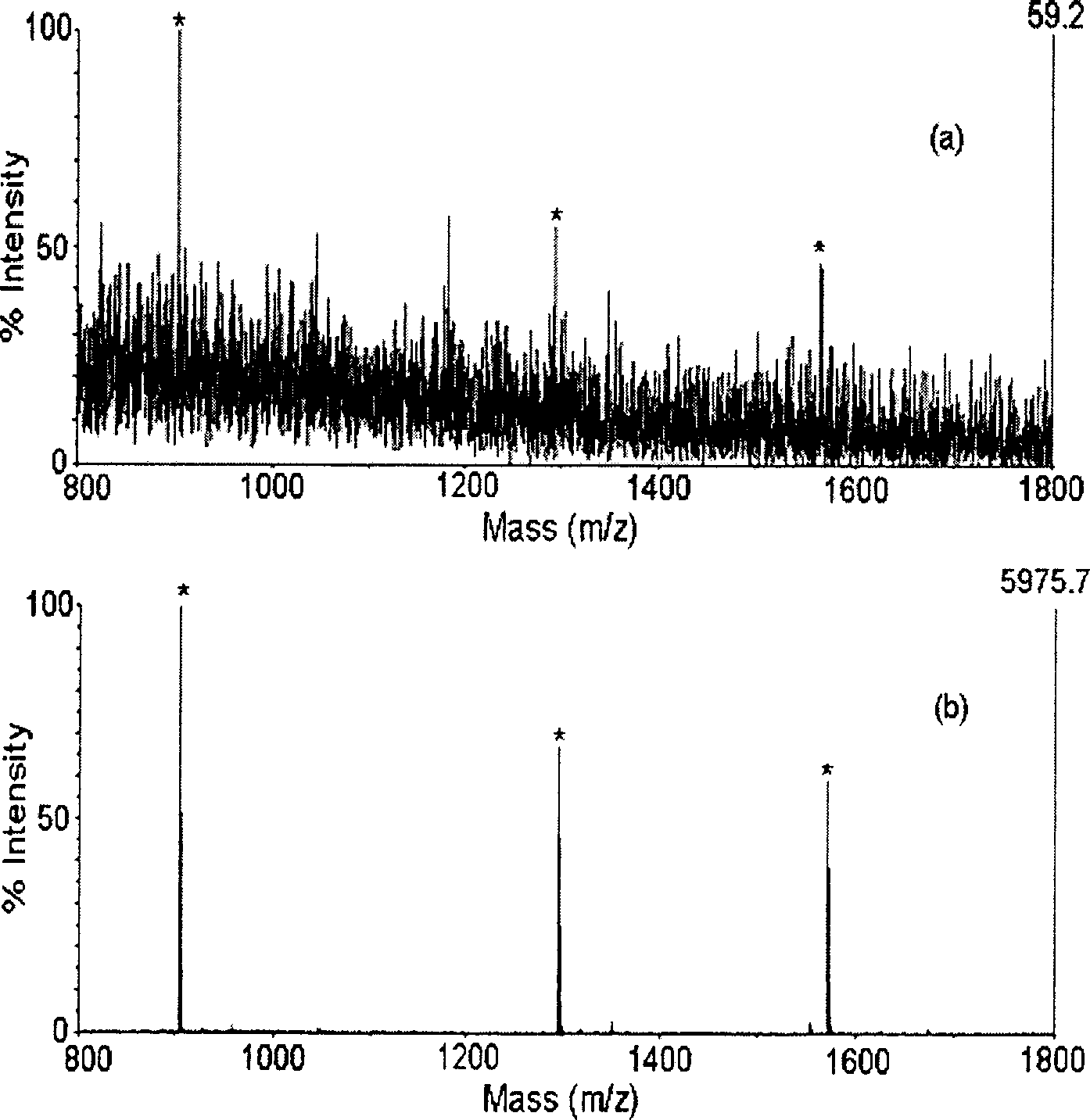
[0027] Prepare 1 fmol / μL level of peptide mixture (standard peptides 1, 2, 3 dissolved in 50% ACN50% H 2O), take 0.5 μL solution and mix it with an equal volume of α-CHCA matrix solution (50% acetonitrile aqueous solution of 0.1% TFA) on the MALDI target plate, carry out MALDI-TOF / MS analysis after drying and crystallization, see figure 2 a. nanoCaCO 3 -PMMA material was incubated in the above solution for 10 minutes, centrifuged for 10 minutes, discarded the supernatant, then took 5 μL of acetonitrile solution containing acetic acid (0.2 μL) to re-mix the stationary phase, centrifuged for 5 minutes, and took the supernatant (final ACN supernatant solution containing peptide / PMMA chain) 0.5 μL and equal volume of α-CHCA matrix solution (50% acetonitrile aqueous solution of 0.1% TFA) were mixed and dried on the MALDI target plate and then MALDI-TOF / MS analysis was carried out. See figure 2 b....
Embodiment 2
[0028] Example 2CaCO 3 -Study on the adsorption of cytochrome c protein by PMMA material
[0029] Adjust the sample solution to 500 fmol / μL cytochrome c protein solution (50% ACN50% H 2 O), other conditions remain unchanged, repeat above-mentioned enrichment experiment. The results obtained can be found in the appendix image 3 shown.
Embodiment 3
[0030] Example 3CaCO 3 -Study on the adsorption of PMMA material to horse myoglobin
[0031] Adjust the sample solution to 600fmol / μL horse myoglobin solution (50%ACN50%H 2 O), other conditions remain unchanged, repeat above-mentioned enrichment experiment. The results obtained can be found in the appendix Figure 4 shown in a and c. Figure 4 b is nano CaCO 3 - PMMA material was incubated in the above solution for 10 minutes and then centrifuged for 10 minutes, then 0.5 μL of the supernatant was mixed with an equal volume of α-CHCA matrix solution (0.1% TFA in 50% acetonitrile aqueous solution) on a MALDI target plate and dried after crystallization Results of MALDI-TOF / MS analysis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com