Quality control method for red sage root and notoginseng preparation
A technology of Sanqi and Danshen, which is applied in the field of HPLC-DAD fingerprint identification of Danshen and Sanqi compound, which can solve the problems of failure to identify at the same time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] 1 Experimental materials
[0087] 1.1 Instruments and equipment:
[0088] Agilent 1100 series high performance liquid chromatography, including G1312A quaternary gradient pump, G1313A autosampler, G1316A column thermostat, G1315A DAD detector, HP Chemstation chromatography workstation (U.S. HP Company); rotary thin film evaporator (Switzerland) Büchi company).
[0089] 1.2 Reagents and reagents:
[0090] Medicinal materials: Danshen (Shaanxi Tasly Plant Pharmaceutical Co., Ltd., Shangluo, Shaanxi), identified by Professor Li Ping as the dry root of Salvia miltiorrhiza Bge. The dry root of Panax notoginseng (Burk.) F.H.Chen.
[0091] 2. Experimental method
[0092] 2.1 Chromatographic conditions
[0093]Chromatographic column: C18 chromatographic column (ZORBAX ODS 4.6×250mm ID, 5μm) and C18 pre-column (4.6×12.5mmID, 5μm); column temperature: 30°C; mobile phase, A is 0.1% phosphoric acid water; B is acetonitrile; A+B=100%, gradient elution: 0-10min, 7-17%B, 10-12mi...
Embodiment 2
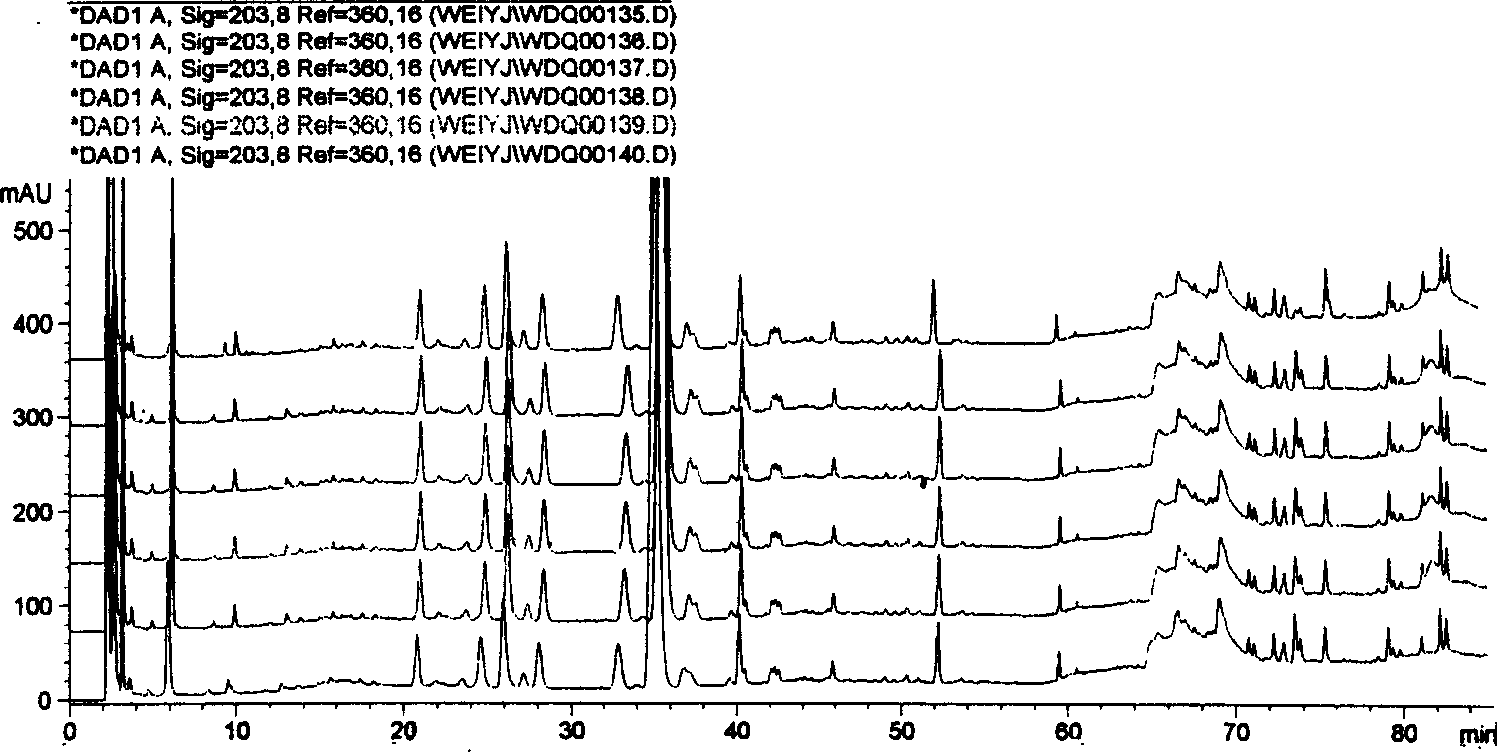
[0098] Take 20 commercially available Danqi Tablets (Hunan Anbang Pharmaceutical Co., Ltd., batch number: 040803), remove the sugar coating and grind them into powder, accurately weigh 0.5g, put in a 25ml brown volumetric flask, and dilute to volume with 70% methanol. Ultrasonic extraction was performed for 30 min, allowed to cool, and 70% methanol was added to make up the weight. The solution was passed through a 0.45 μm membrane filter. Other conditions are with embodiment 1. Inject 10 μl for chromatographic analysis, get the HPLC-DAD fingerprint of seven tablets of Dandan, see Image 6 .
Embodiment 3
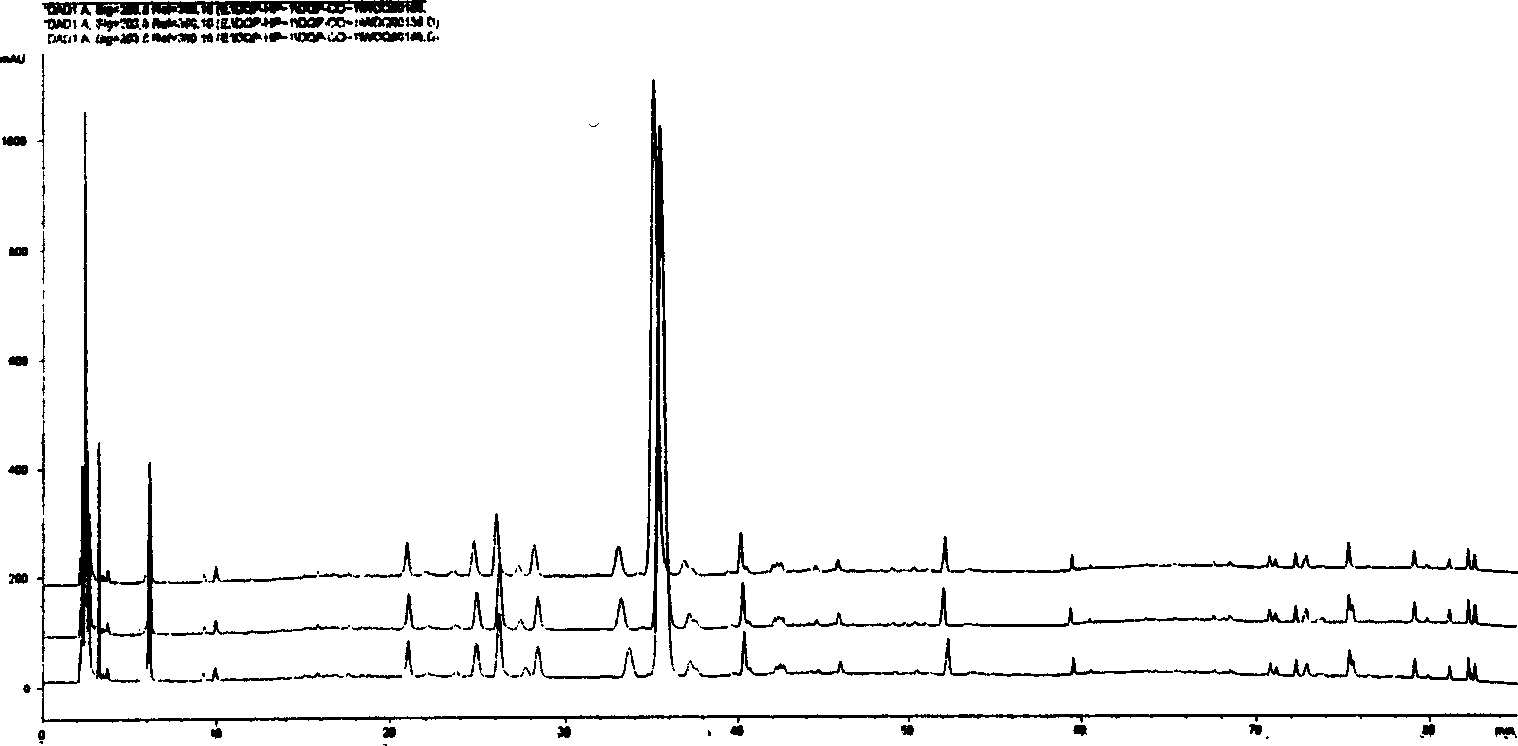
[0100] Get commercially available Compound Danshen Tablets (Guangdong Baiyunshan Pharmaceutical Factory, batch number: 03121024; Nanjing Tongrentang Pharmaceutical Co., Ltd., batch number: 040602; Zhejiang Huqing Yutang Pharmaceutical Co., Ltd., batch number: 050308) 20 tablets, ground into powder, and accurately weighed Take 0.5g, put it in a 25ml brown volumetric flask, and dilute to volume with 70% methanol. Ultrasonic extraction was performed for 30 min, allowed to cool, and 70% methanol was added to make up the weight. The solution was passed through a 0.45 μm membrane filter. Other conditions are with embodiment 1. Inject 10 μl of sample for chromatographic analysis, and obtain the HPLC-DAD fingerprints of Compound Danshen Tablets from 3 manufacturers, see Figure 7-9 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com