Optimal placement of a robust solvent/detergent process post viral ultrafiltration of an immune gamma globulin
A technology of immunoglobulin and non-ionic detergent, which is applied in the direction of immunoglobulin, virus/bacteriophage, virus, etc., and can solve problems such as destruction and protein denaturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
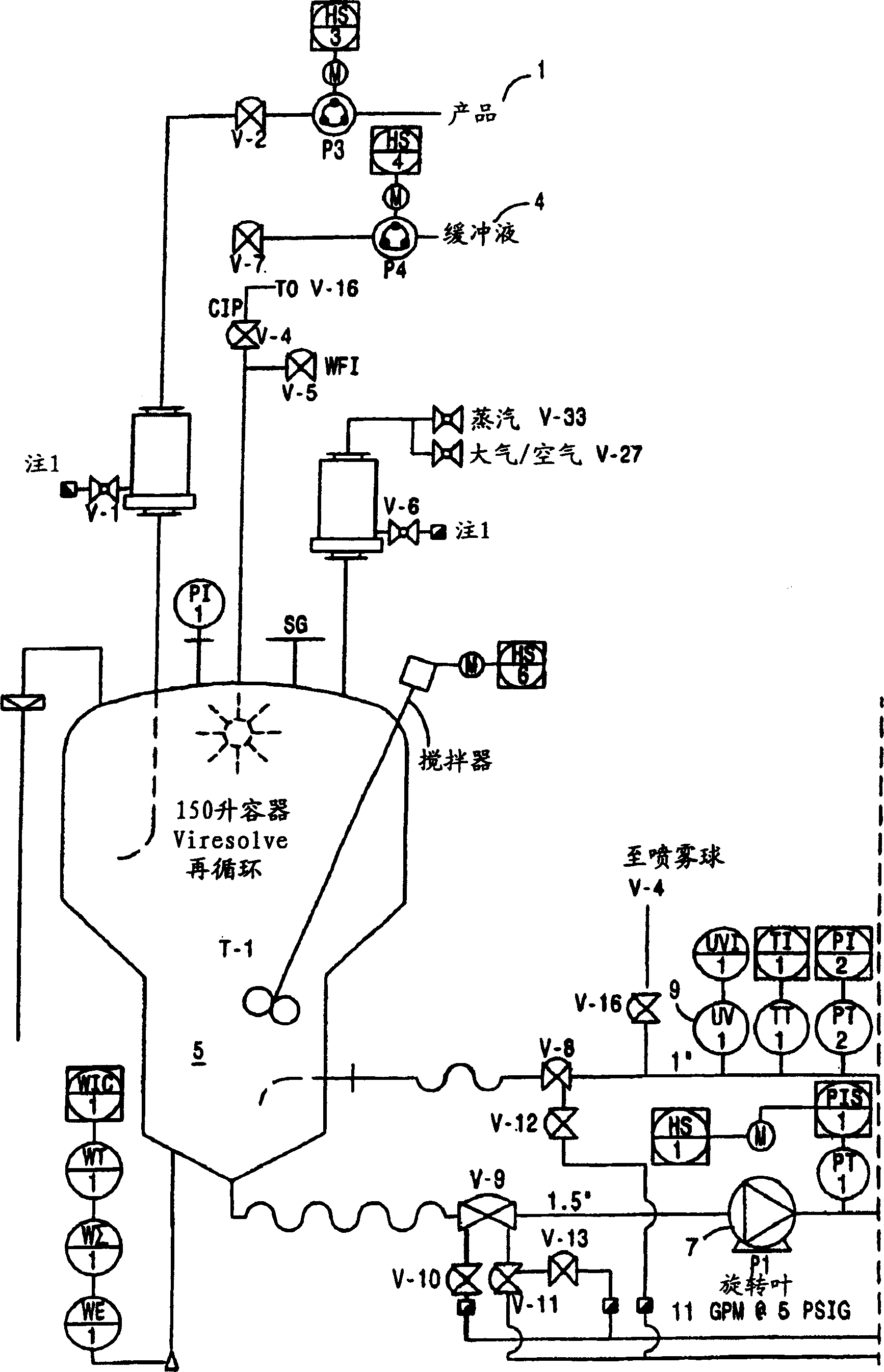
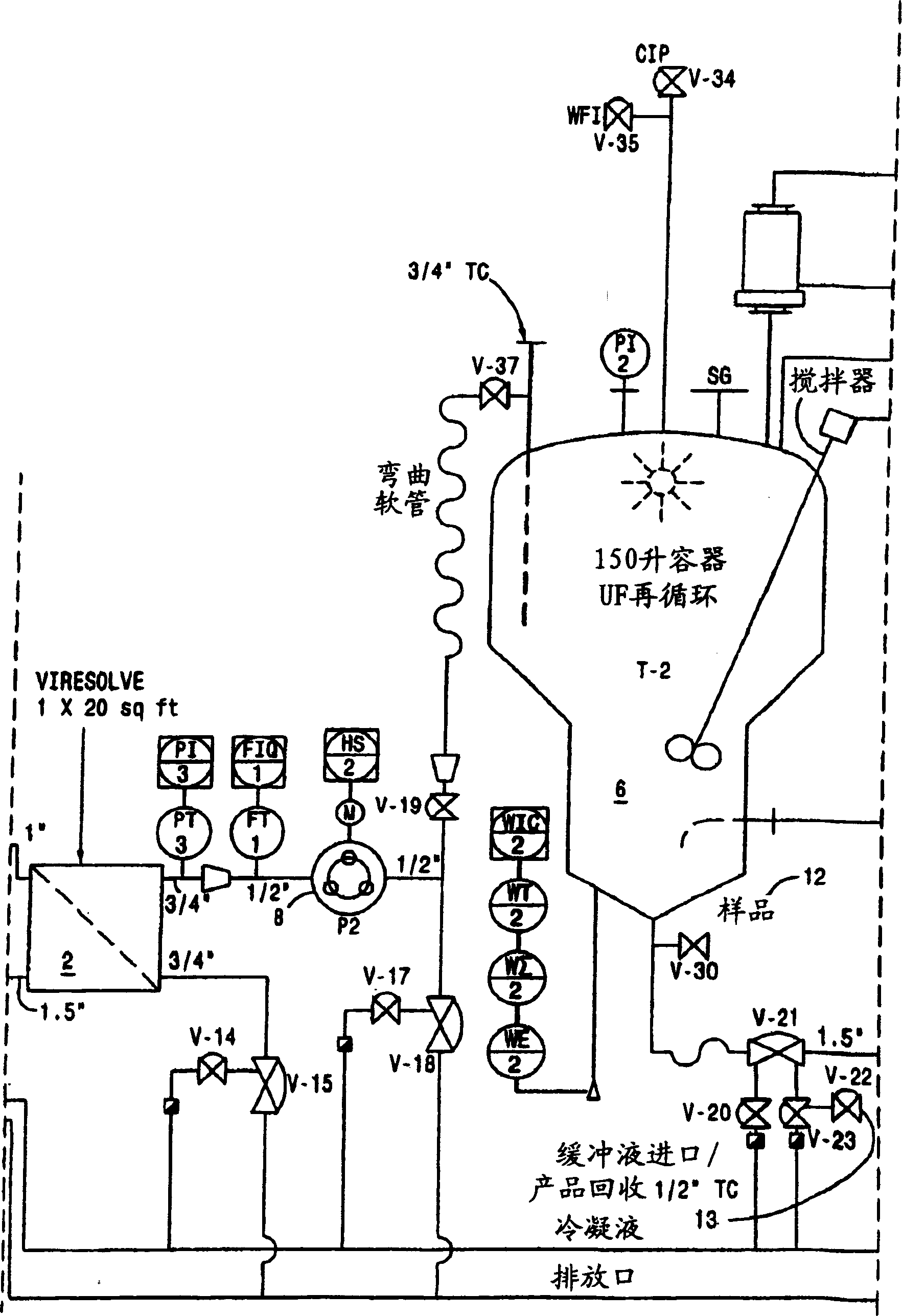
[0174] Virus-depleted RhoGAM was prepared by ultrafiltration as described in U.S. Patent No. 6,096,872 , with the following improvements:
[0175] 6.802 Kg of Rho(D) immunoglobulin purified to step "Pellet II Slurry" by the modified Cohn purification method was resuspended in 20.406 L of Water for Injection (WFI), U.S.P., and cooled to 4°C. The mixture was vortexed (no foaming) for 4 hours and stored at 4°C until use.
[0176] After the SIP procedure, a Viresolve-180R module (Millipore Corporation) (20 stacks) for a volume of pellet II resuspended in a volume of approximately 27.208 L was installed. The Pellicon CIP / SIP module was replaced with 2 Biomax-50 cassettes installed. Viresolve-180 modules were chlorinated and cleaned as described above. The Biomax-50 membrane was rinsed with WFI, U.S.P. Benzalkonium chloride (Roccal) was measured on the last permeated rinse sample; the benzalkonium chloride content was 8 ppm. Diffusion tests were performed on Biomax-50 cartrid...
Embodiment 1A
[0216] The 150 mM NaCl-glycine buffer used in Example 1 was prepared as follows:
[0217] Calculate the appropriate buffer volume to prepare as follows:
[0218] [Resuspended slurry volume (L)×10L]×2+60=approximate buffer volume to be prepared
[0219] [27.208L×10L]×2+60=604.16L buffer to be prepared
[0220] Determine and measure the amount of material needed to add to a calibrated depyrogenated container:
[0221] Material
required concentration
×Batch size
= required amount
NaCl
Polysorbate 80
8.87g / L
15.01g / L
0.02g / L
604.16L
604.16L
604.16L
5,358.90g
9,068.44g
12.08g
[0222] Rinse the weighed Polysorbate container several times with a total of approximately 2 L of Water for Injection, U.S.P., adding an aliquot of each wash to the volume of each batch to make up to 604 L. Determine the quantities of the following materials:
[0223] Material
required concentrat...
Embodiment 2
[0228] Using S / D and sorbent in RhoGAM virus inactivation in
[0229] UF Feasibility Study
[0230] Materials and methods
[0231] squeeze-D immunoglobulin
[0232] Human immunoglobulins are obtained by a fully modified Cohn-Oncley fractionation method, (see U.S. Patent No. 6,096,872, and Examples 1 and 1A herein), followed by nanofiltration using a Viresolve 180 size exclusion filter to prepare RhoGAM Ultra-Filtered Rh(D) Immunoglobulin (Human), (Ortho-Clinical Diagnostics, Raritan, NJ). The material is stored under sterile conditions at 2°C-8°C until use.
[0233] Preparation of virus
[0234] Viruses were prepared as titered stock cultures prior to spiking into immunoglobulins. Stocks of bovine viral diarrhea virus (BVDV), pseudorabies virus (PRV), and West Nile virus (WNV) (strain NIAID V-554-110-522, ATCC, Manassas, VA) were prepared according to industry standard procedures. with cultures.
[0235] TCID on samples collected by the Orho Clinical-Diagnosti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com