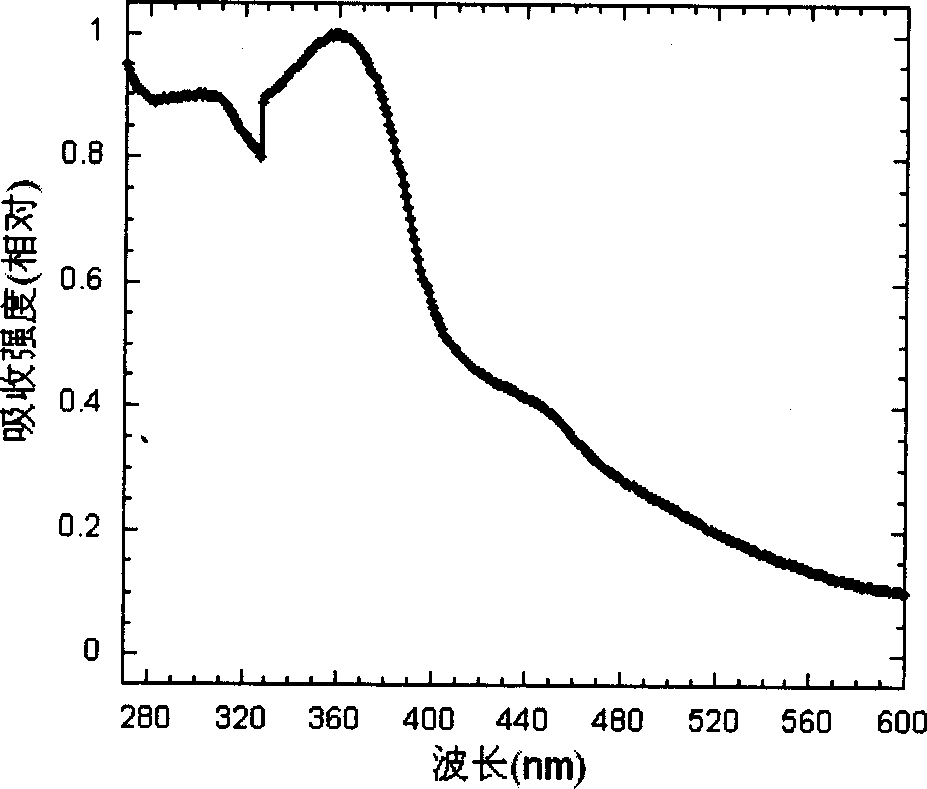
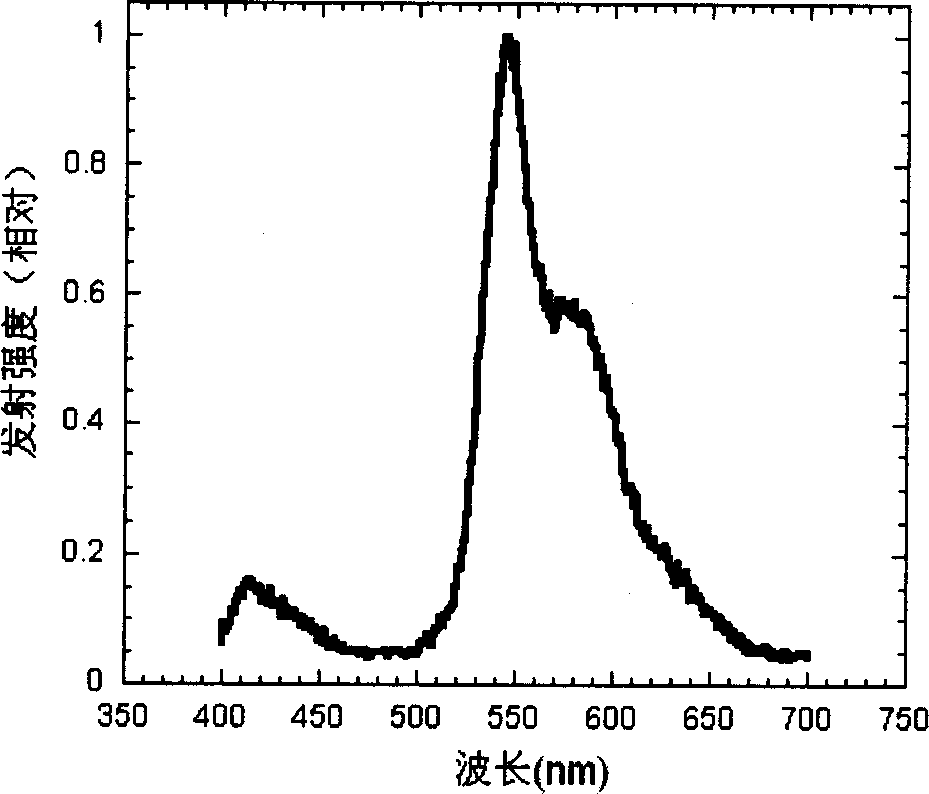
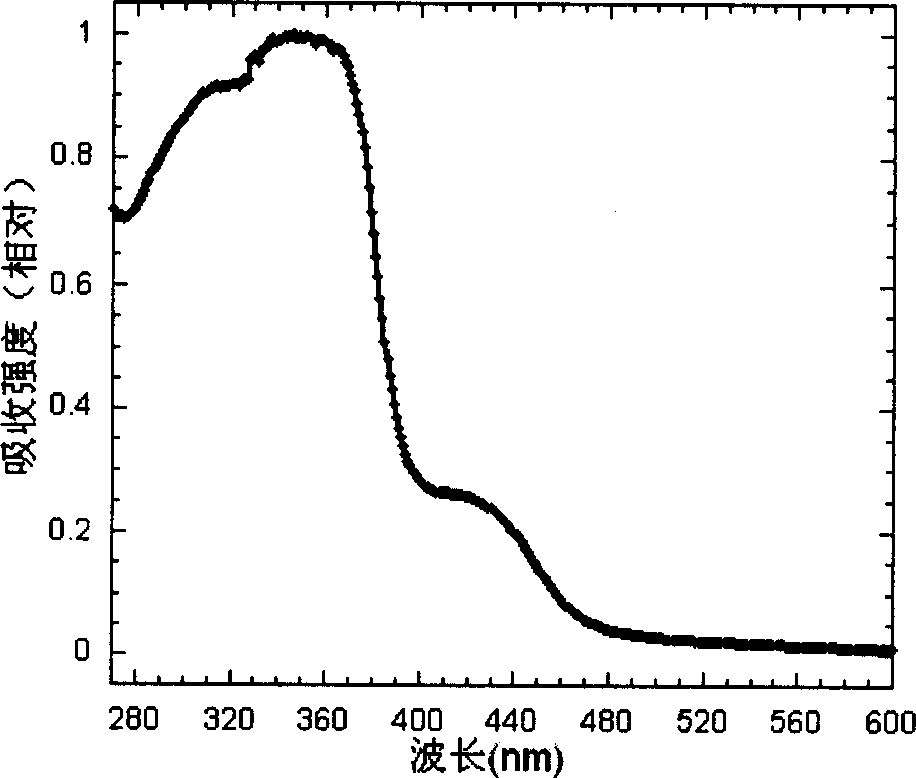
Cyclic metallic platinum compounding agent electrofluor scence material containing triaryl amine functional redical
A phosphorescent material and electrophosphorescence technology, applied in luminescent materials, electroluminescence light sources, electric light sources, etc., can solve the problems of difficulty in improving luminous efficiency and achieve good carrier injection and excellent recombination performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of 2-(4-aminophenyl)pyridine
[0027]
[0028] In a 100mL three-necked flask, add 4.0g (20mmol) 2-(4-nitrophenyl)pyridine and 40mL ethanol, heat to 50°C, add 0.2g 5% Pd-C, and then add 4mL 80 % hydrazine hydrate and 0.2g 5% Pd-C, reflux reaction 4hr. After most of the ethanol was evaporated, 15 mL of water was added to precipitate a white solid, which was filtered by suction, washed with water, and dried in vacuum to obtain 3.4 g of white crystals with a yield of 98.8%. Melting point (m.p). 96-97°C (literature value [9] 97-98°C). Infrared spectrum (IR) (KBr, cm -1 ): 3452, 3306 (N-H), 3190 (Ar-NH2 combination peak), 1631, 1606, 1522, 1470 (aromatic ring skeleton), 836 (1,4-disubstituted benzene). Proton nuclear magnetic spectrum (1HNMR) (400MHz, CDCl 3 ) δppm: 8.62 (d, 1H), 7.83 (d, 2H), 7.70 ~ 7.61 (m, 2H), 7.14 ~ 7.09 (t, 1H), 6.76 (d, 2H), 3.81 (s, 2H).
Embodiment 2
[0030] Synthesis of N,N-bis(4-tert-butylphenyl)-4-(2'-pyridyl)aniline (BuPhNPPy)
[0031]
[0032] 1.0g (5.9mmol) 2-(4-aminophenyl) pyridine, 3.8g (14.6mmol) 4-tert-butyl iodobenzene, 6.6g anhydrous potassium carbonate, 1.5g activated copper powder, 0.4g dibenzo -18-Crown-6 and 20mL of o-dichlorobenzene were added to a 100mL three-neck flask, under the protection of nitrogen, heated to reflux for 21hr, cooled, suction filtered, and o-dichlorobenzene was recovered by vacuum distillation, and the residue was washed with 200-300 mesh silica gel As the stationary phase, dichloromethane was used as the eluent, and separated by column chromatography to obtain 1.2 g of white solid with a yield of 46.8%. m.p.228~230℃. 1HNMR (400MHz, CDCl3 ) δppm: 8.54 (d, 1H), 7.85 (d, 2H), 7.64 ~ 7.70 (m, 2H), 7.28 (d, 4H), 7.04 ~ 7.17 (m, 7H), 1.32 (s, 18H).
Embodiment 3
[0034] Synthesis of N, N-diphenyl-4-(2'-pyridyl)aniline (PhNPPy)
[0035]
[0036] 1.5g (8.9mmol) 2-(4-aminophenyl) pyridine, 2.5mL (21.9mmol) iodobenzene, 9.9g (71.7mmol) anhydrous potassium carbonate, 2.3g activated copper powder, 0.6g dibenzo- Add 18-crown-6 and 30mL o-dichlorobenzene into a 100mL three-necked flask, heat and reflux for 24hrs under the protection of nitrogen, cool, filter with suction, and recover o-dichlorobenzene by distillation under reduced pressure. As the stationary phase, dichloromethane was used as the eluent and separated by column chromatography to obtain 1.5 g of white solid with a yield of 51.7%. m.p.178-179°C. 1HNMR (400MHz, CDCl 3 )δppm: 8.73~8.62(d, 1H), 7.88~7.86(d, 2H), 7.73~7.65(m, 2H), 7.29~7.25(t, 4H), 7.18~7.05(m, 7H), 7.06~ 7.02(t, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Light emitting area | aaaaa | aaaaa |
| Maximum luminance | aaaaa | aaaaa |
| Maximum luminescence wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com