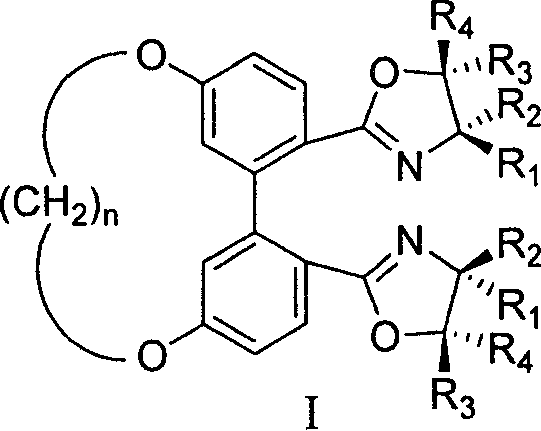
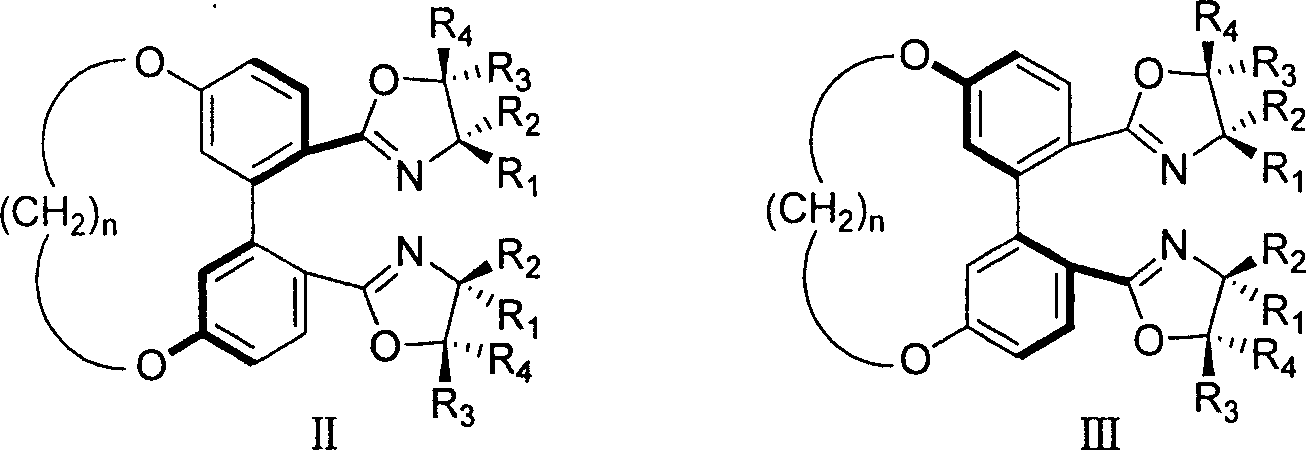
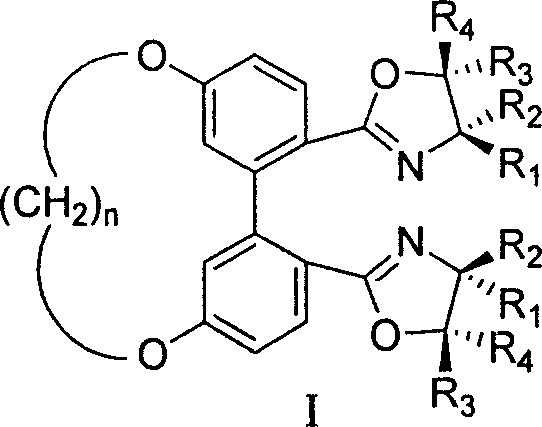
5,5' bit connected 1,1'-diphenyl kind axle chirality ligand
An axial chirality and ligand technology, applied in the field of 1, compounds, can solve problems such as limiting the rotation angle of biphenyl, and achieve the effect of good application prospect, high reactivity and stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The following examples will help to further understand the present invention, but do not limit the content of the present invention.
[0025] (1) Preparation of compound IV from 2-bromo-4-methoxybenzophenone
[0026] Under ice bath, slowly drop bromine (2.8mL, 35.1mmol) into NaOH (4.5g, 111.4mmol) aqueous solution (10mL), stir for 10min, then add 2-bromo-4-methoxybenzophenone (2.1 g, 9.2mmol) was dropped into the above reaction solution and stirred at room temperature for 15h. After the reaction finishes, add aqueous sodium sulfite to eliminate unreacted sodium hypobromite. The neutral compound was separated by extraction with ethyl acetate, and the aqueous phase was acidified with 6N hydrochloric acid in an ice bath, and a large amount of white solid appeared. Filtration afforded IV as a white solid (2.0 g, 95%).
[0027] 1 H NMR (CDCl 3 , 300MHz) 8.05 (d, J = 8.7Hz, 1H, Ar-H), 7.23 (d, J = 2.7Hz, 1H, Ar-H), 6.91 (dd, J = 8.7, 2.7Hz, 1H, Ar- H), 3.87(s, 3H, OCH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com