Method of producing zinc vitriol using indium extraction raffinate and zinc oxide
A technology of zinc sulfate heptahydrate and indium raffinate, which is applied in the direction of zinc sulfate, can solve side effects and other problems, and achieve the effects of accelerating filtration speed, saving raw materials, and saving time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
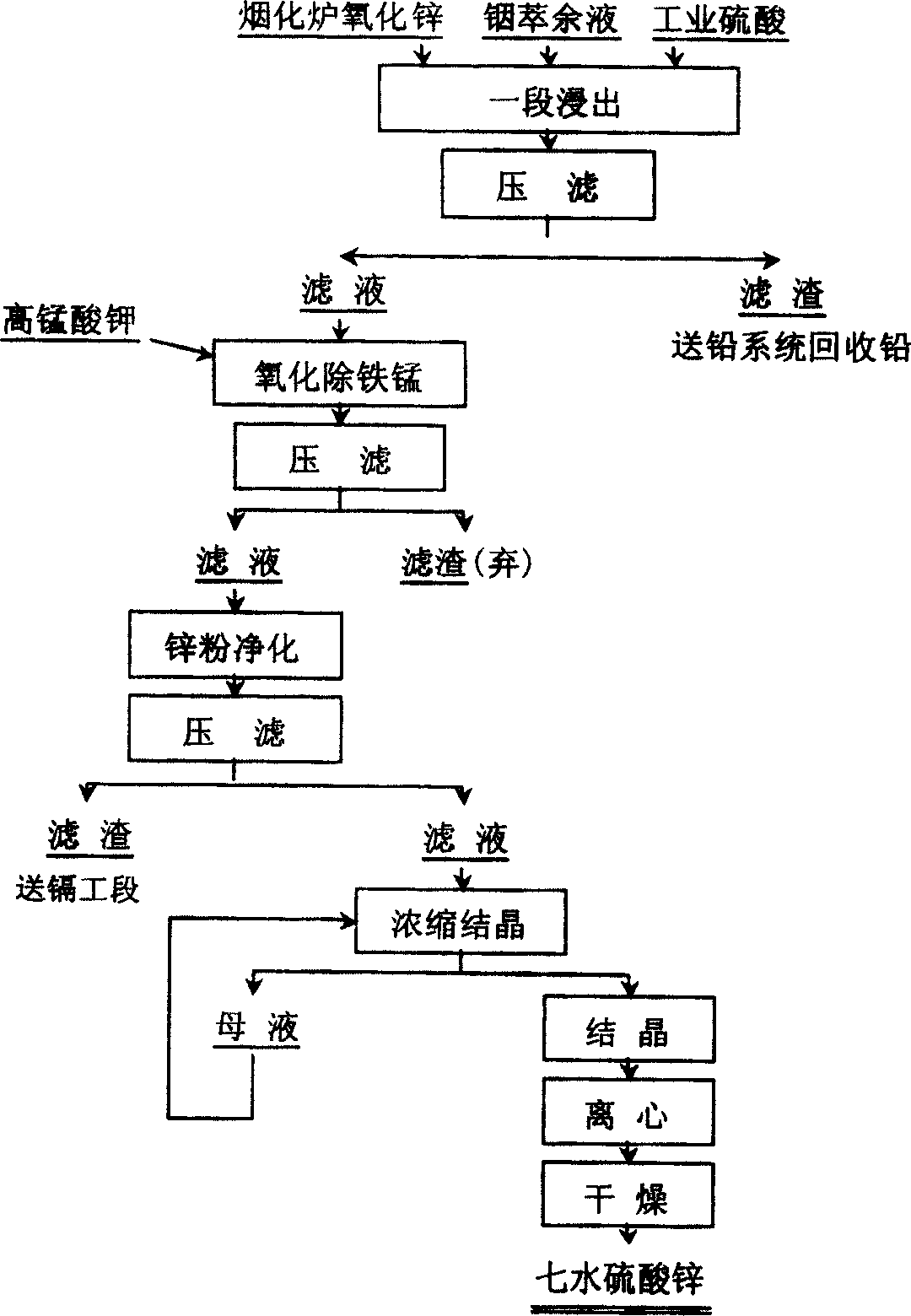
[0024] Produce zinc sulfate heptahydrate with the present invention, contain zinc 43.93g / l, sulfuric acid 52.22g / l, organic matter P in the indium raffinate 204 0.07g / l, arsenic 2.43g / l, iron 1.64g / l, manganese 1.75g / l, copper 0.51g / l, cadmium 0.72g / l, etc. The production steps are as follows:
[0025] 1. Preparation of leaching solution: Calculate the amount of indium raffinate to be added, the amount of industrial sulfuric acid with a concentration of 93% and the amount of zinc oxide in the lead fuming furnace according to the amount of zinc and sulfuric acid contained in the indium raffinate, and use the calculation results to Indium raffinate and sulfuric acid are prepared leach solution, obtain zinc: the leach solution of the molar ratio of sulfuric acid=1:1.2;
[0026] 2. Zinc oxide leaching step: raise the temperature of the leaching solution. After the temperature of the solution rises to 70°C, continue to heat up and slowly add zinc oxide to the lead fuming furnace f...
Embodiment 2
[0033] Produce zinc sulfate heptahydrate by the method of embodiment 1 with the present invention, difference is:
[0034] Indium raffinate contains zinc 30.93g / l, sulfuric acid 80.00g / l, organic matter P 204 0.07g / l, arsenic 2.53g / l, iron 1.84g / l, manganese 1.54g / l, copper 0.76g / l, cadmium 0.88g / l, etc.
[0035] In the leach solution preparation step, zinc: the mol ratio of sulfuric acid=1: 1.5, sulfuric acid concentration is 98%; Potassium permanganate is industrial pure in the purifying step, directly uses with solid granular state; When the filtrate is concentrated to specific gravity in the concentrating step, be 1.60g / cm 3 Put it into the crystallization tank; in the crystallization step, centrifuge when the solution temperature is cooled to 40°C.
Embodiment 3
[0037]Produce zinc sulfate heptahydrate with the present invention by the method for embodiment 1, difference is: contain zinc 60.93g / l, sulfuric acid 50.22g / l, organic matter P in the indium raffinate 204 0.05g / l, arsenic 2.43g / l, iron 1.88g / l, manganese 2.09g / l, copper 0.65g / l, cadmium 0.53g / l, etc.
[0038] In the leach solution preparation step, zinc: the mol ratio of sulfuric acid=1: 1, and sulfuric acid concentration is 95%; Potassium permanganate is industrial pure in the purifying step, directly uses with solid granular state; When filtrate is concentrated to specific gravity in the concentration step, be 1.60g / cm 3 Put it into the crystallization tank; in the crystallization step, centrifuge when the solution temperature is cooled to 35°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com