Transparent non-water proton conductive material of side group type azacyclic polymer and its preparing method
A technology of nitrogen heterocyclic compound and proton conduction, which is applied in the direction of organic material conductors, non-metallic conductors, color-changing fluorescent materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The raw materials used are as follows:
[0028] ITO conductive glass, 80Ω / □, product of Dongtai Vacuum Coating Engineering Co., Ltd.
[0029] Tungsten oxide, analytically pure, provided by Shanghai Chemical Reagent Company.
[0030] Nickel oxide, analytically pure, provided by Shanghai Chemical Reagent Company.
[0031] Poly(5-vinyltetrazole) was synthesized using polyacrylonitrile reference literature (Gaponik PN, Ivashkevich OA, Karavai VP, Lesnikovich AI, Chernavina NI. Die Angew Makromol Chem1994, 219:77) with a molecular weight of 18131.5.
[0032] N, N-dimethylformamide (DMF), a product of Shanghai Zhenxing Chemical No. 1 Plant.
[0033] 85wt% phosphoric acid, product of Shanghai United Chemical Plant.
[0034] The ratio of raw materials used is as follows:
[0035] 100 parts of poly(5-vinyltetrazole) (number of moles of repeating units)
[0036] 100 parts of phosphoric acid (number of moles)
[0037] Poly(5-vinyltetrazole) was fully dissolved in N,N-dimethy...
Embodiment 2
[0044] The raw materials used are as follows:
[0045] Poly(N-vinyltriazole) is obtained by polymerization of N-vinyltriazole monomer by free radical polymerization.
[0046] 85wt% phosphoric acid, product of Shanghai United Chemical Plant.
[0047] The ratio of raw materials used is as follows:
[0048] Poly(N-vinyltriazole) 100 parts (number of moles of repeating units)
[0049] 25 parts of phosphoric acid (number of moles)
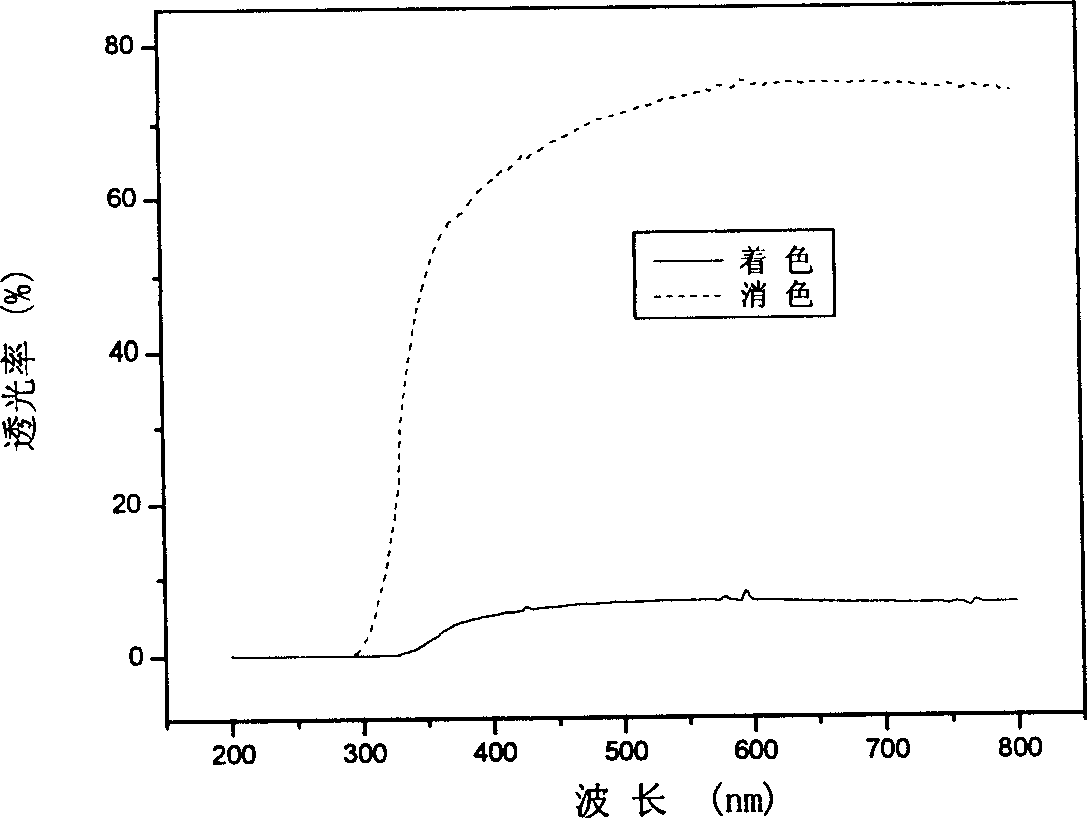
[0050] The preparation process of other layers and device assembly of the all-solid-state electrochromic device is similar to that shown in Example 1. The electrical conductivity of the obtained composite film was as Figure 4 As shown, the light transmittance of the monolayer film is as Figure 5 shown. The electrochromic properties of the device and image 3 Similar to shown.
Embodiment 3
[0052] The ratio of raw materials used is as follows:
[0053] 100 parts of poly(5-vinyltetrazole) (number of moles of repeating units)
[0054] 50 parts of phosphoric acid (number of moles)
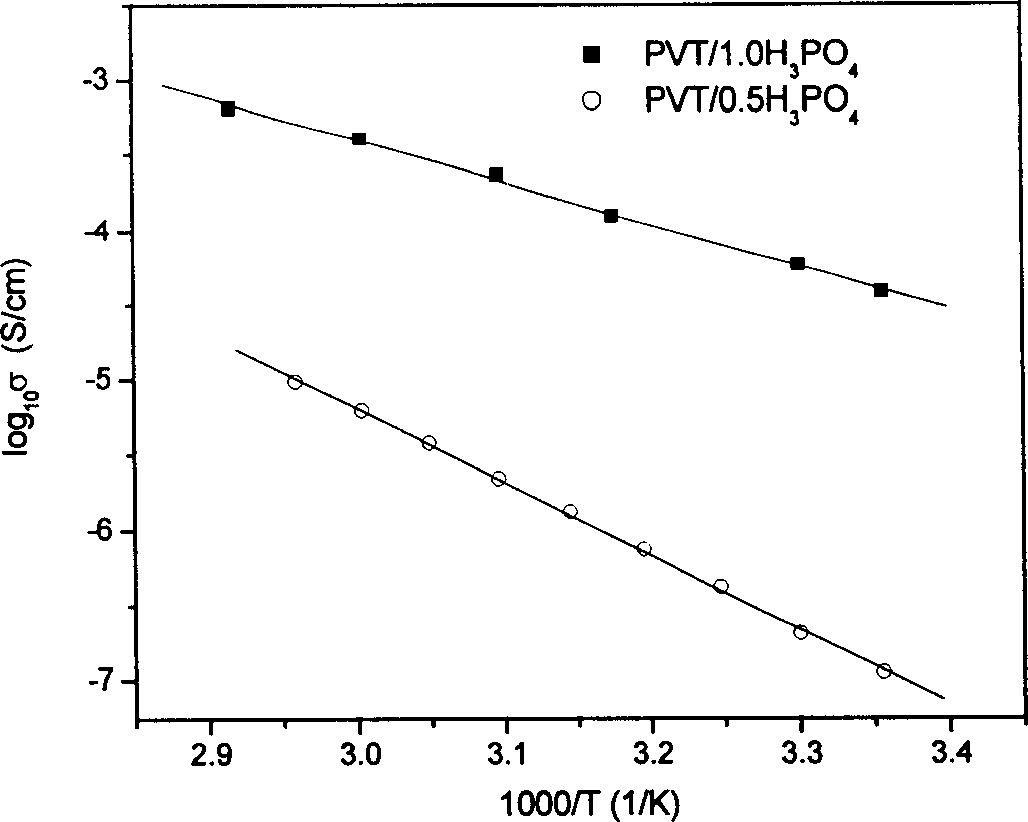
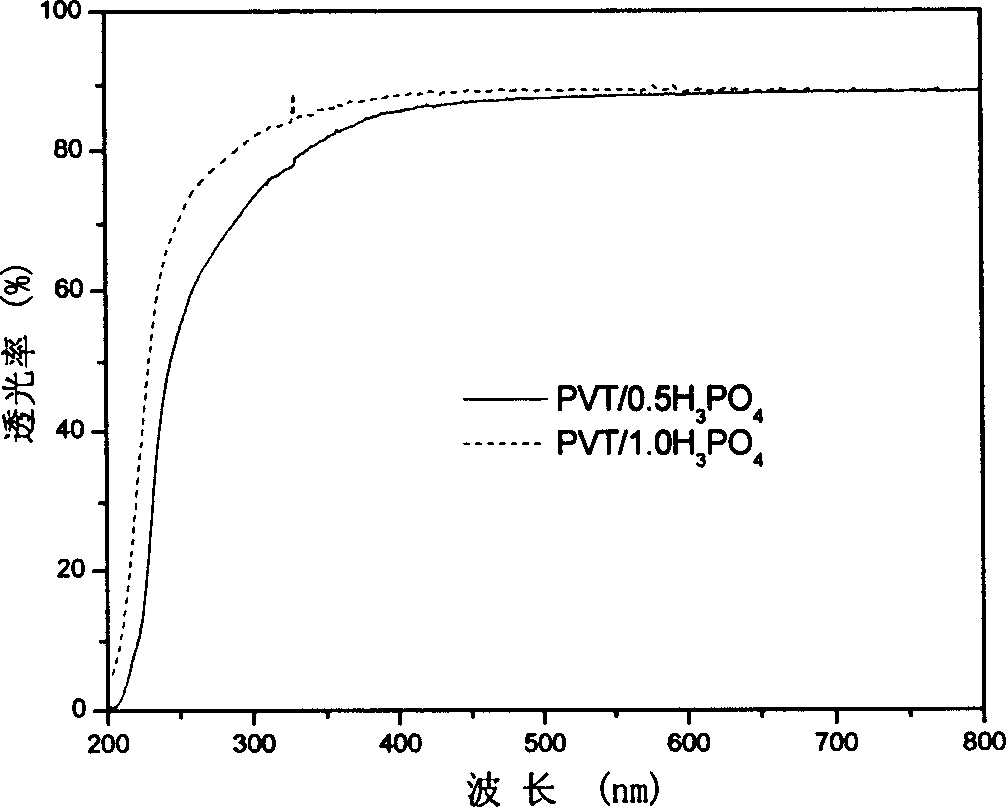
[0055] The preparation process of other layers and device assembly of the all-solid-state electrochromic device is similar to that shown in Example 1. The electrical conductivity of the obtained composite film was as figure 1 As shown, the light transmittance of the monolayer film is as figure 2 As shown, the electrochromic performance of the device is related to image 3 Similar to shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com