Polypeptide combined with T cell surface co-stimulation molecule CD137 and its use
A costimulatory molecule, cell surface technology, applied in the direction of peptides, etc., can solve the problem of unclear key sequences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: Screening of CD137-binding peptides from a phage random heptapeptide library
[0059] materials, reagents
[0060] Random heptapeptide phage display library (Ph.D.7 TM Phage Display Peptide Library Kit) was purchased from New England Biolabs, USA, and the titer of the phage was 2.0×10 13 pfu / ml with a complexity of 2.8 × 10 9 The transformant, the host bacterium is E.coli ER2738.
[0061] Recombinant human CD137 was purchased from R&D Company.
[0062] IPTG and Xgal were purchased from Takara Company.
[0063] PEG8000 was purchased from Promega.
[0064] The sequencing primer 5'-CCC TCA TAG TTA GCG TAA CG-3' was synthesized by Shanghai Boya Company.
[0065] Coating solution: 0.1M NaHCO3 pH 8.6
[0066] Blocking solution: 0.5% BSA-NaHCO3
[0067] Eluent: 0.2M Glycine-HCl (pH 2.2), 1mg / ml BSA
[0068] Neutralizing solution: 1M Tris-HCl (pH 9.1)
[0069] Wash solution: 0.1% TBST, 0.5% TBST
[0070] experiment
[0071] 1.1 Phage random heptapeptide li...
Embodiment 2
[0086] Embodiment 2: ELISA experiment detects the affinity of peptide and CD137
[0087] materials, reagents
[0088] Horseradish peroxidase (HRP)-labeled anti-M13 phage monoclonal antibody (HRP / anti-M13) is a product of Pharmacia.
[0089] experiment
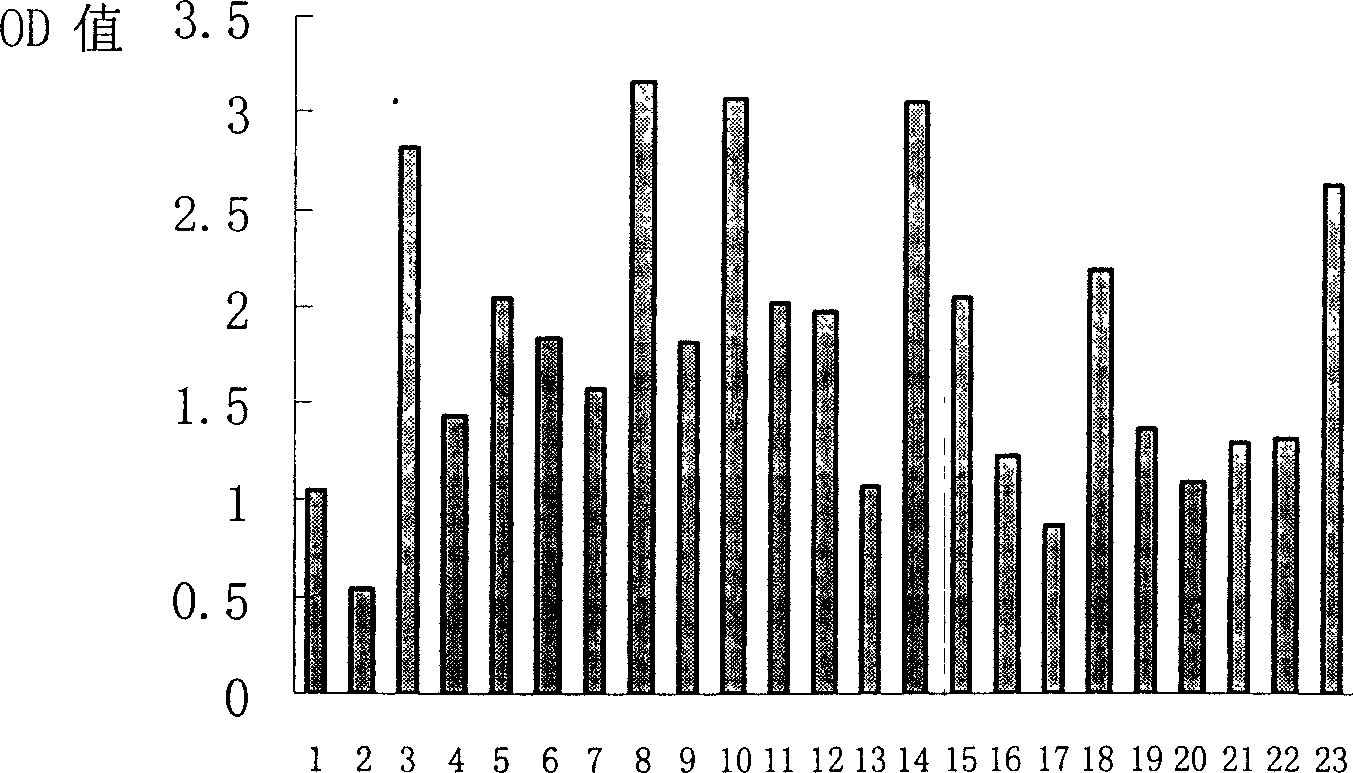
[0090] 2.1 Sandwich ELISA to detect the binding of panned phage-displayed polypeptides to target molecules
[0091] When amplifying the above-mentioned phage plaques for DNA sequencing, the remaining supernatant containing phage plaques was stored at 4°C. For each plaque clone to be identified, inoculate a tube of host bacteria ER2738 in 20ml LB medium, and culture at 37°C until slightly turbid. Add 5 μl of phage supernatant to each tube of ER2738 culture medium, and incubate at 37°C for 4.5hrs. The culture was transferred to a centrifuge tube and centrifuged for 10 min. Transfer the supernatant to a fresh centrifuge tube and centrifuge again. Take 80% of the supernatant in a fresh centrifuge tube and add 1 / 6 volume of PE...
Embodiment 3
[0099] Example 3: Phage Displayed Polypeptide Inhibits Anti-CD137 Antibody-Induced T Cell Proliferation in Vitro
[0100] materials, reagents
[0101] PHA was purchased from Kerui Biotechnology Company
[0102] 3 H-TdR was purchased from China Institute of Atomic Energy
[0103] Anti-CD137Ab was purchased from R&D Company.
[0104] experiment
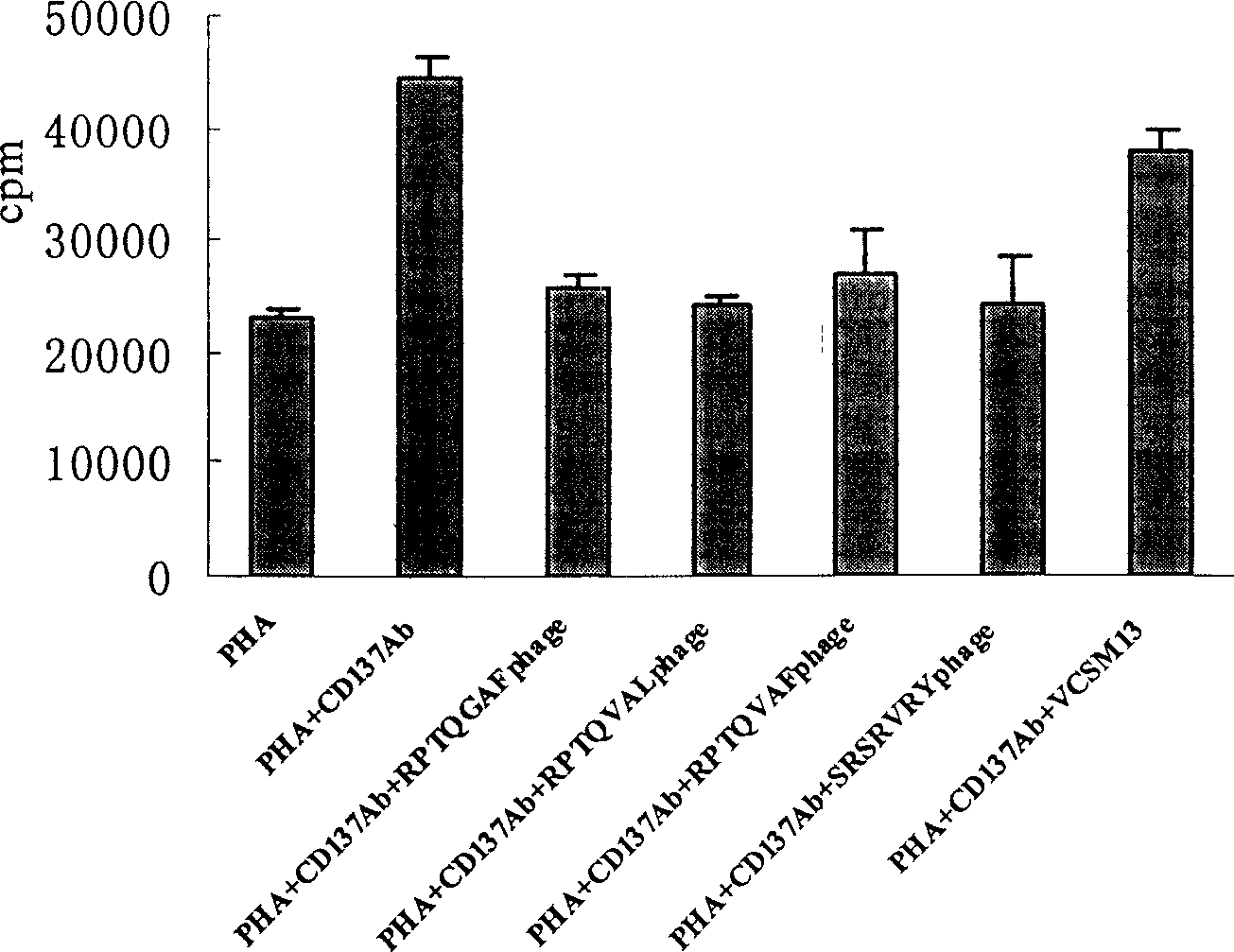
[0105] For the screened phage clones containing consensus sequences, 1×10 12 Phages were added to a 96-well plate and irradiated with ultraviolet light for three hours to inactivate the phages infectivity and viability, but still maintain the displayed polypeptides. The effect of each phage-displayed polypeptide on the proliferation response of human peripheral blood lymphocytes stimulated by PHA and anti-CD137Ab was detected in vitro. At the same time, an irrelevant phage VCSM13 was used as a control.
[0106] Routine separation of human peripheral blood lymphocytes, adjust the cell concentration to 1×10 6 / ml, add 200ul per we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com