Medicine for treating parkinson's disease and Parkinson's syndrome and its prepn
A Parkinson's disease, Parkinson's technology, applied in the field of traditional Chinese medicine, can solve problems such as on-off phenomenon, end-of-dose phenomenon, and ineffectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example I
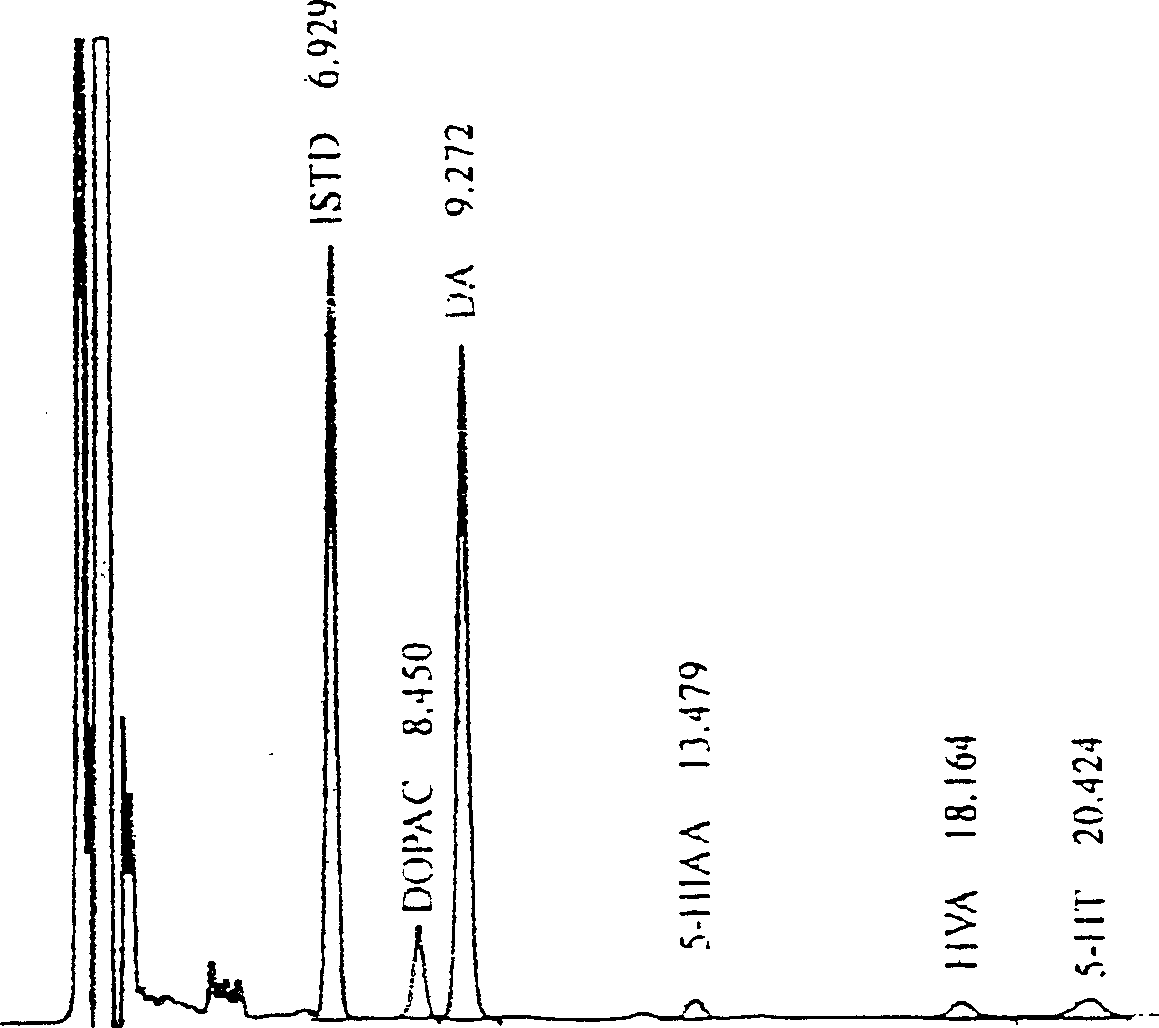
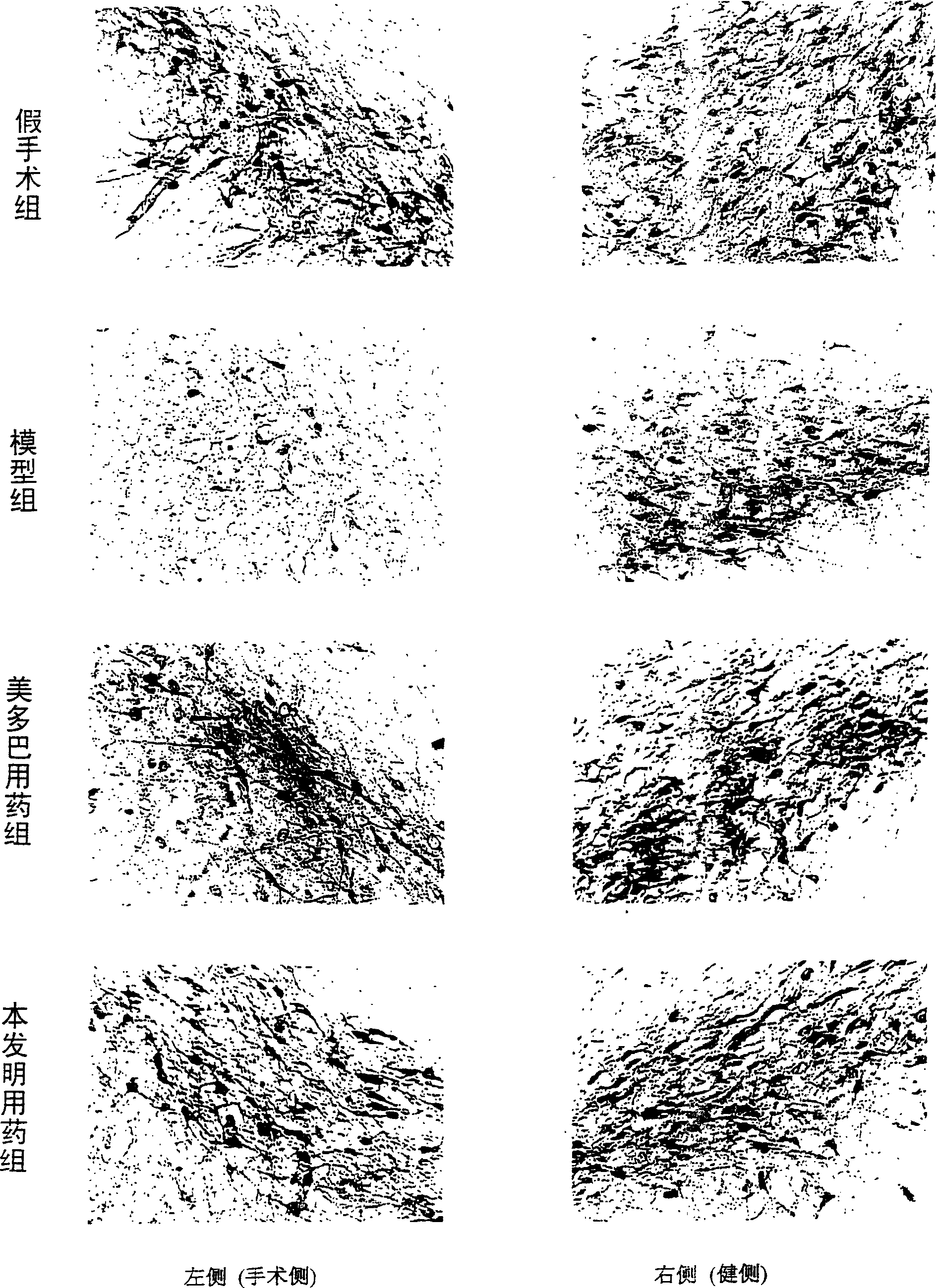
[0037] [Experimental example 1] the pharmacological action of medicine of the present invention to unilateral nigrostriatal pathway damage rat model
[0038] This experimental example investigates the motor function of the drug of the present invention on the Parkinson's disease-like model rat caused by unilateral nigrostriatal pathway damage, the level of lipid peroxide in the rat cortex, the content of dopamine in the striatum, and the number of neurons in the substantia nigra. Impact.
[0039] Experimental Materials:
[0040] 1. Animal: SD rat, male, weighing 200±20g
[0041] 2. Reagents and instruments
[0042] Reagents: 6-hydroxydopamine (6-hydroxydapamine, 6-OHDA), octane sulfonate sodium salt (octylsulfate sodium salt, OSA), dopamine (Dopamine, DA), dihydroxyphenylacetic acid (dihydroxyphenylacetic acid, DOPAC), dihydroxy Benzylamine (3,4-dihydroxybenzylamine, DHBA), homovanillic acid (homovanillic acid, HVA), thiobarbituric acid (TBA), ethylenediaminetetraacetic aci...
experiment example 2
[0119] [Experimental example 2] the pharmacological effect of drug granules of the present invention on drug-induced muscle tremor model mice
[0120] In this experiment example, two drug-induced muscle tremor models were selected: arecoline tremor and oxygenated tremorine tremor. Record the latency period and duration of tremor in animals to observe the effect of the drug particles of the present invention on the tremor behavior of the drug.
[0121] Experimental Materials
[0122] 1. Animal: ICR mouse, male, clean grade, body weight 18±2g.
[0123] 2. Reagents: Oxotremorin, Arecoline.
[0124] experimental method:
[0125] 1. Animal grouping and drug administration
[0126] The mice were randomly divided into groups, and the mice in the normal control group were given distilled water for continuous intragastric administration (0.3ml / only) for 3 weeks before giving a single intraperitoneal injection of 0.9% normal saline (0.3ml / only); the mice in the model group were give...
experiment example 3
[0153] [Experimental Example 3] Pharmacological effects of drug granules of the present invention on MPTP-induced PD-like model mice
[0154] Experimental Materials:
[0155] 1. Animals: C57BL mice, half female and half male, clean grade, weighing 18±2g.
[0156] 2. Reagents and instruments: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, MPTP).
[0157] Instrument: CS-2 Mouse Autonomic Activity Programmer.
[0158] experimental method:
[0159] 1. Establishment of MPTP-induced Parkinson's disease mouse model: MPTP was directly dissolved in 0.9% sterile saline, and its final concentration was adjusted to 2 mg / ml. Animals in the model group were injected with MPTP20mg / kg continuously intraperitoneally for a total of 8 days.
[0160] 2. Grouping and administration: C57BL mice were randomly divided into groups. Normal control group: After intragastric administration of distilled water for 3 weeks, the same dose of normal saline as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com