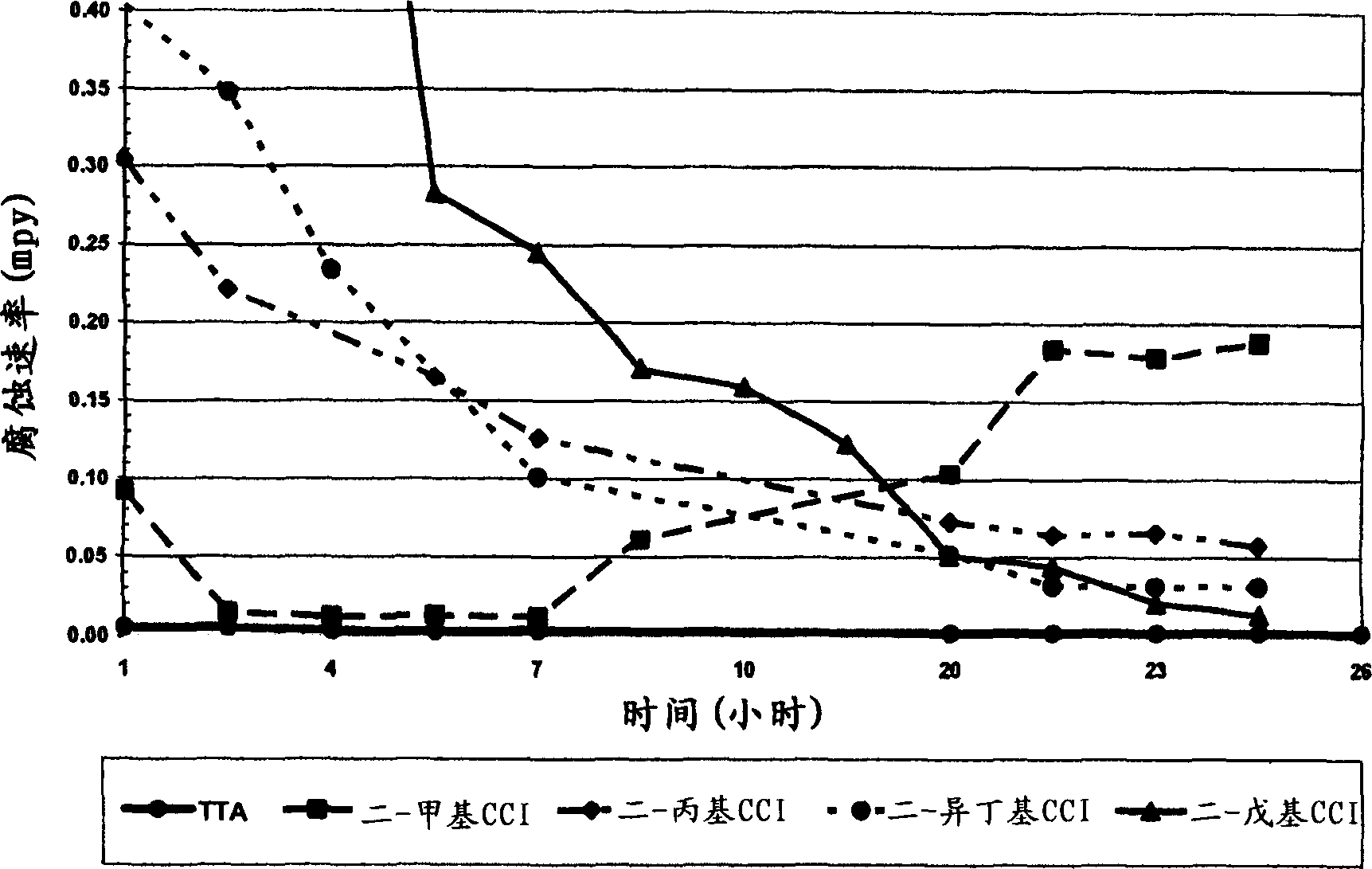
Sulfur based corrosion inhibitors
By forming a durable protective barrier, sulfur-based corrosion inhibitors solve the problem of triazole corrosion inhibitors being sensitive to chlorine degradation and requiring residual inhibitors, achieving anti-corrosion effects without residue, reducing costs and monitoring frequency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Preparation of sodium dimethyldithiocarbamate in aqueous solution
[0127] A clean and dry four-necked 500 mL flask was charged with 59.6 g of tap water, 39.0 g (0.52 mol) of 60% dimethylamine in water, and a large stir bar. Stirring was started and the flask was fitted with a condenser, thermocouple and heating mantle. A 25 mL addition funnel was charged with 38.0 g (0.50 mol) of carbon disulfide and the addition funnel was loaded onto the reaction flask. A 50 mL addition funnel was charged with 40.0 g (0.50 mol) of 50% sodium hydroxide, which was then loaded onto the reaction flask. The reaction was then heated to 30°C with stirring.
[0128] When the contents of the reactor reached 30°C, the addition of carbon disulfide was started at a slow drop rate. After 5 minutes, sodium hydroxide was also added at a slow dropwise rate. The additions were adjusted so that the reaction temperature did not exceed 45°C, and after about 1 hour both additions were complete. The ...
Embodiment 2
[0130] Preparation of sodium diethyldithiocarbamate in aqueous solution
[0131] A clean dry four-neck 500 mL flask was charged with 113 g of tap water, 19.0 g (0.26 mol) of diethylamine, and a large stir bar. Stirring was started and the flask was fitted with a condenser, thermocouple and heating mantle. A 25 mL addition funnel was charged with 19.0 g (0.25 mol) of carbon disulfide, which was then loaded onto the reaction flask. A 50 mL addition funnel was charged with 20.0 g (0.50 mol) of 50% sodium hydroxide and the addition funnel was loaded onto the reaction flask. The reaction was then heated to 30°C with stirring.
[0132] When the contents of the reactor reached 30°C, the addition of carbon disulfide was started at a slow drop rate. After 5 minutes, sodium hydroxide was also added at a slow dropwise rate. The additions were adjusted so that the reaction temperature did not exceed 45°C, and after about 1 hour both additions were complete. The reaction was then boil...
Embodiment 3
[0134] Preparation of sodium dipropyldithiocarbamate in aqueous solution
[0135]A clean dry four-necked 500 mL flask was charged with 189 g of tap water, 36.9 g (0.365 mol) of dipropylamine (Aldrich, 99%), and a large stir bar. Stirring was started and the flask was fitted with a condenser, thermocouple and heating mantle. A 25 mL addition funnel was charged with 26.6 g (0.35 mol) of carbon disulfide, which was then loaded onto the reaction flask. A 50 mL addition funnel was charged with 28.0 g (0.35 mol) of 50% sodium hydroxide and the addition funnel was loaded onto the reaction flask. The reaction was then heated to 30 °C with stirring.
[0136] When the contents of the reactor reached 30°C, the addition of carbon disulfide was started at a slow drop rate. After 5 minutes, sodium hydroxide was also added at a slow dropwise rate. The additions were adjusted so that the reaction temperature did not exceed 45°C, and after about 1 hour both additions were complete. The re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electric potential / voltage | aaaaa | aaaaa |
| composition ratio | aaaaa | aaaaa |
| composition ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com