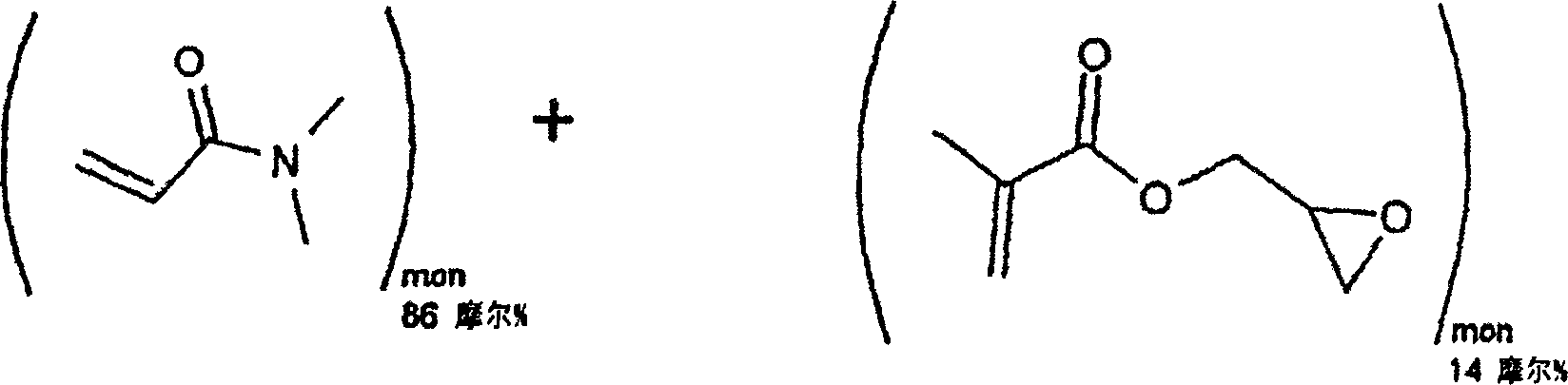Intraocular lenses with modified surface
A technology of intraocular lens and hydrophilic monomer, which is applied in the field of surface modification of polymeric materials, and can solve problems such as bad IOL turbidity and IOL turbidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
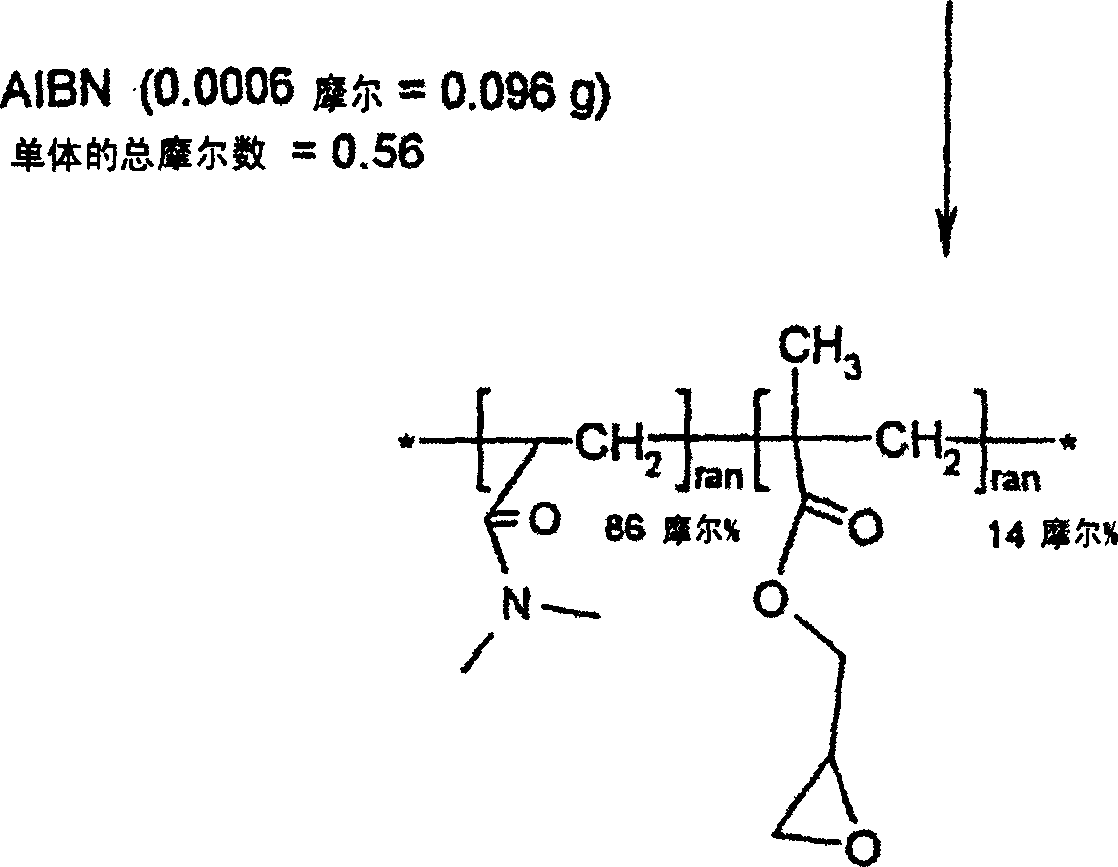
[0029] Embodiment 1: Reactive hydrophilic N, the synthesis of N-dimethylacrylamide (DMA) and glycidyl methacrylate (GMA) copolymer
[0030]
[0031] Molecular weight = 99.13 Molecular weight = 142.16
[0032] Molecular formula = C 5 h 9 NO molecular formula = C 7 h 10 o 3
[0033] 48g, 0.48 mole 12g, 0.08 mole
[0034]
[0035] Distilled N,N-dimethylacrylamide (DMA, 48 grams, 0.48 moles), distilled glycidyl methacrylate (GMA, 12 grams, 0.08 moles), 2,2'-azobis Isobutyronitrile (AIBN, 0.096 g, 0.0006 mol) and toluene (600 ml) were added to a 1 liter reaction flask. The reaction vessel was equipped with a magnetic stirrer, cooler, temperature controller, and nitrogen inlet. Nitrogen was bubbled through the solution for 15 minutes to remove all dissolved oxygen. The reaction vial was then heated to 60°C for 20 hours under an insulated blanket of nitrogen. The reaction mixture was then slowly added to 6 liters of ether with good mechanical stirring. The reactive p...
Embodiment 2
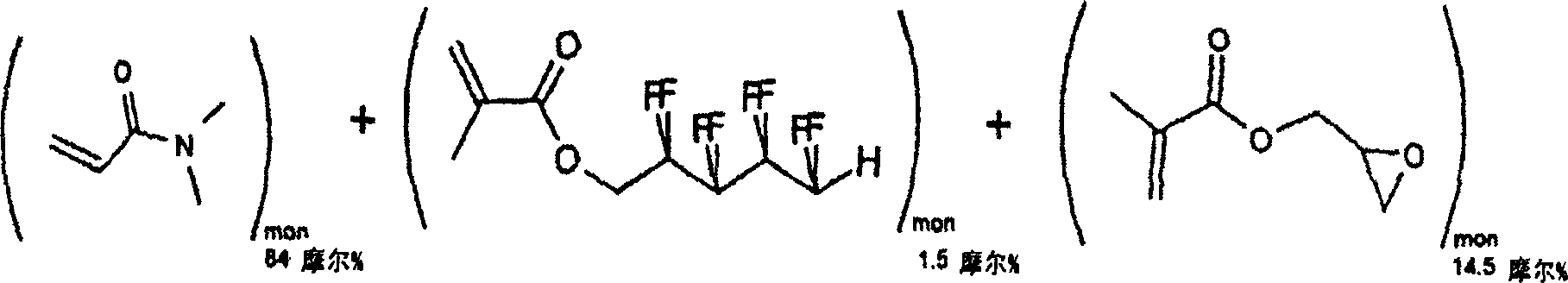
[0038] Example 2: Synthesis of N, N-dimethylacrylamide (DMA), 1H, 1H, 5H-octafluoropentyl methacrylate (OFPMA) and water-soluble reactivity of glycidyl methacrylate (GMA) polymer
[0039]
[0040] Molecular weight = 99.13 Molecular weight = 300.15
[0041] Molecular weight = 142.16
[0042] Molecular formula = C 5 h 9 NO molecular formula = C 9 h 6 f 8 o 2
[0043] Molecular formula = C 7 h 10 o 3
[0044] 16g, 0.16 mole 1g, 0.003 mole
[0045] 4g, 0.028 mole
[0046]
[0047] Distilled N, N-dimethylacrylamide (DMA, 16 g, 0.16 moles), 1H, 1H, 5H-octafluoropentyl methacrylate (OFPMA, 1 g, 0.003 moles), distilled Glycidyl methacrylate (GMA, 4 g, 0.028 mol), 2,2'-azobisisobutyronitrile (AIBN, 0.03 g, 0.00018 mol) and toluene (300 ml) we...
Embodiment 2f
[0050] Example 2f: Synthesis of N, N-dimethylacrylamide (DMA), 1H, 1H, 5H-octafluoropentyl methacrylate (OFPMA), glycidyl methacrylate (GMA) and polyethylene glycol 1000 Reactive hydrophilic copolymer of monomethyl ether methacrylate (PEGMA)
[0051]
[0052] Molecular weight = 99.13 Molecular weight = 300.15
[0053]Molecular weight = 142.16
[0054] Molecular formula = C 5 h 9 NO molecular formula = C 9 h 6 f 8 o 2
[0055] Molecular formula = C 7 h 10 o 3
[0056]
[0057] Molecular weight = 1113.35
[0058] Molecular formula = C 51 h 100 o 25
[0059]
[0060] N,N-dimethylacrylamide (DMA, 8 grams, 0.08 moles), 1H, 1H, 5H-octafluoropentyl methacrylate (OFPMA, 1 gram, 0.003 moles) obtained by distillation were directly used (used as received)), distilled glycidyl methacrylate (GMA, 4 g, 0.028 mol), polyethylene glycol 1000 monomethyl ether methacrylate (PEGMA, 8 g, 0.007 mol), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| stretchability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com