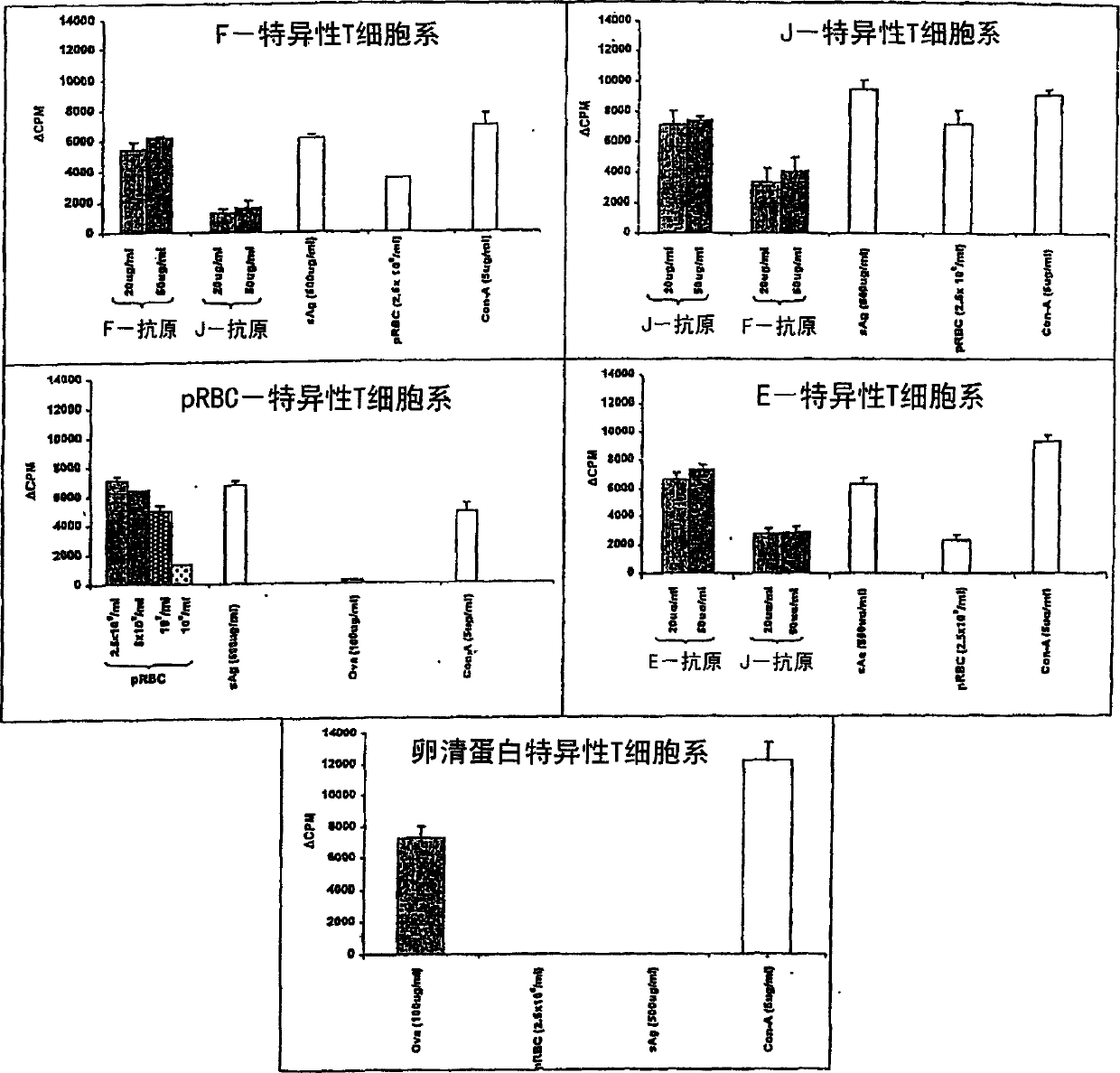
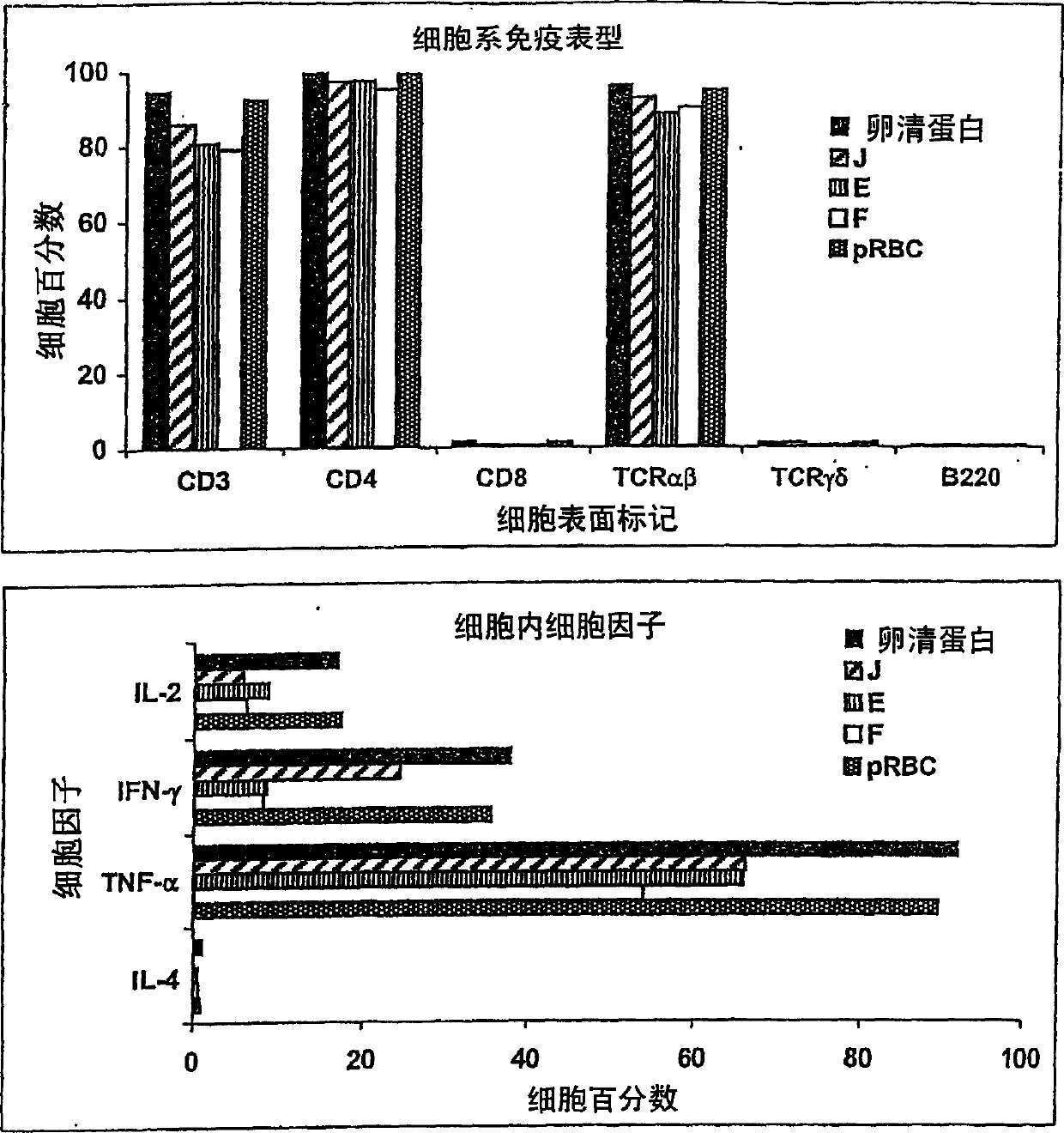
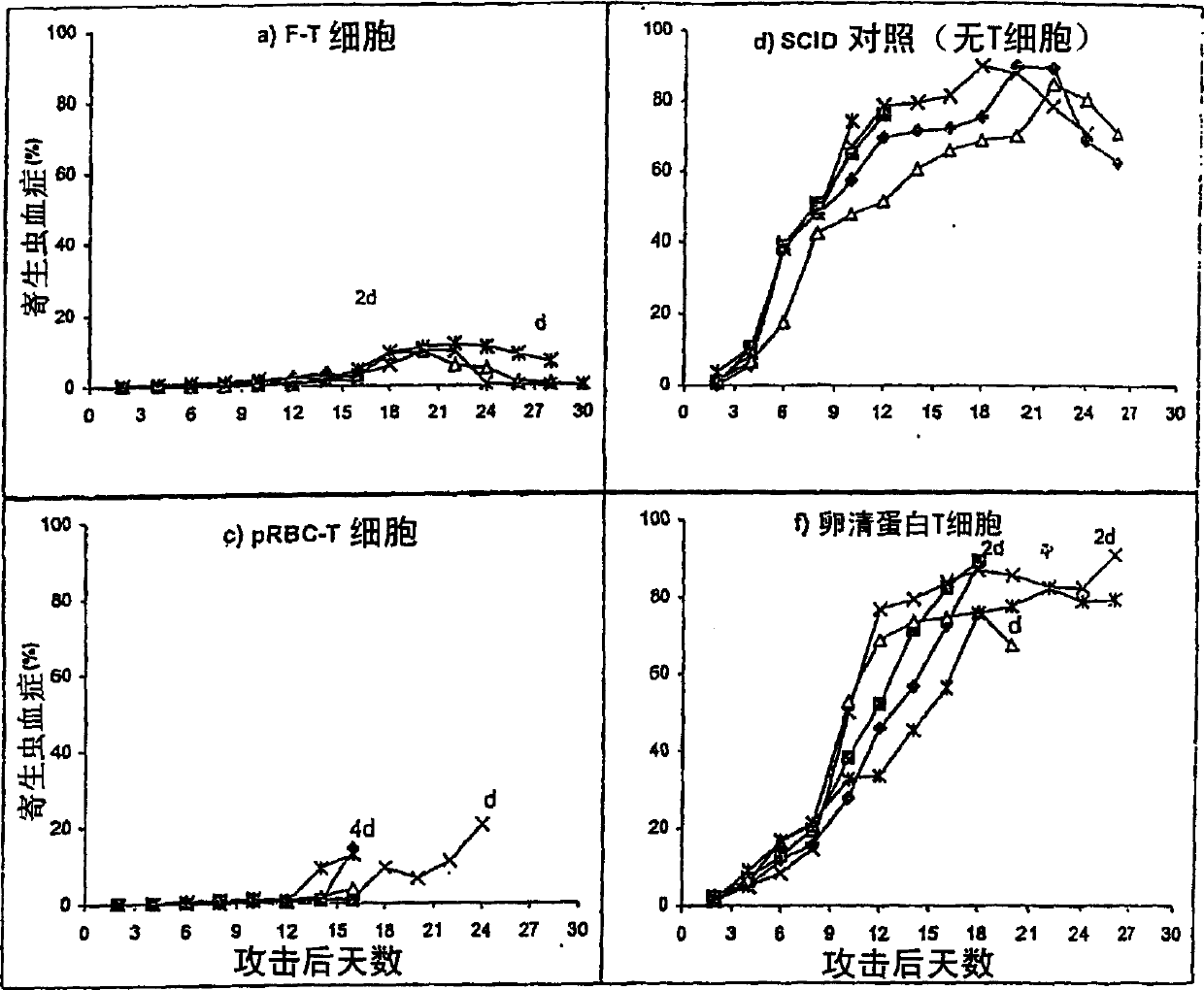
Anti-protozoal vaccine
A technology of immunotherapy and immunogenic fragments, applied in the field of immunotherapy of protozoal diseases, which can solve the problems of the complex life cycle of Plasmodium and unidentified T cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Example 1 Identification of HXGPRT as an immunogen in Plasmodium yoelii
[0132] 1. Materials and methods
[0133] mouse
[0134] 4-6 week-old normal BALB / c mice, athymic BALB / c nude mice and BALB / c SCID mice were purchased from Animal Resources Center (Perth, WA, Australia) and kept under specific pathogen-free conditions (specific- Pathogen-free) were kept in the animal facility (animal facility) of QIMR. At 6-8 weeks of age they were used for testing.
[0135] parasite
[0136] Rodent Plasmodium yoelii 17XNL was carried out between infected and uninfected mice by the intraperitoneal route (i.p.) 6 Alternate passages of parasite-infected red blood cells (pRBC) / mice were maintained. After 3 or 4 passages, stable parasites were then frozen and stored in liquid nitrogen. Cryopreserves were subjected to three alternate passages before being used for experimental challenge infection. Parasites were collected from blood obtained by cardiac puncture and tail docking a...
Embodiment 2
[0186] Example 2 Mapping the T cell epitope of HGXPRT
[0187] The data in Example 1 have established that Plasmodium HGXPRT may play a role in modulating protection against malaria infection. Since mammals also express HGXPRT, and mammalian HGXPRT is quite homologous to parasite HGXPRT (Fig. 6), it was important to identify the minimal non-homologous regions of the protein that might be targets of protective T cells. These regions may be used as malaria vaccine candidates.
[0188] To this point, 24 linear overlapping peptides spanning HGXPRT were designed to define protein regions containing protective T cell epitopes (Figure 7). Various strains of mice were immunized with the full-length P. falciparum HGXPRT protein (PfHGXPRT) or peptide pools from PfHGXPRT to determine protein regions that generate proliferative T cell responses. Afterwards, these regions (corresponding to the peptides) were then used to immunize mice to determine whether the corresponding T cells could ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com