Antimalarial and anti-babesiosis agent and pharmaceutical compositions contg. same
A composition and medicine technology, applied in the application field in the preparation of antimalarial medicines, can solve the problems of increased cost, indecision in treatment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
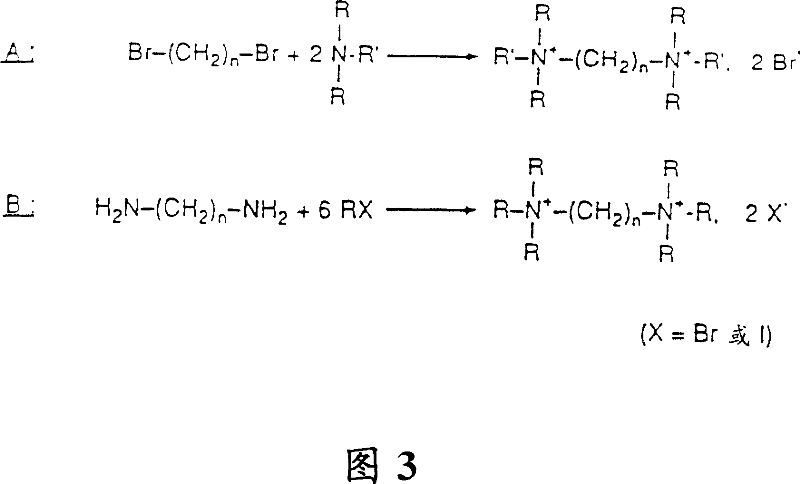
[0092] Example 1: N,N'-dimethyl-N,N'-diethyl-N,N'-dipropyl-1,14-tetradecanediammonium, dibromide
[0093] 35.6 g (0.1 mol) of 1,14-dibromotetradecane and 11.1 g of N-ethyl-N-methyl-1-propylamine were added to 200 ml of ethanol. Reflux for 8 hours until the reaction is complete, followed by thin-layer chromatography on silica gel plates in a solvent system such as propanol / pyridine / acetic acid / water. The solution was then evaporated and the residue was recrystallized from an isopropanol / ethyl acetate mixture. The title derivative is obtained in the form of crystals, m.p. = 185 / 190°C.
[0094] No commercially available α-ω dibromo derivatives can be synthesized from lower dibromoalkane derivatives with 4 carbon atoms by malonic acid synthesis. As an example, the synthesis of 1,14-dibromotetradecane (a, b, c and d) from dibromodecane will be described below:
[0095] a) Synthesis of ethyl 2,13-diethoxycarbonyl-1,14-tetradecanedioate
Embodiment 2
[0104] Example 2: N,N'-dimethyl-N,N'-diethyl-N,N'-dipropyl-1,15-pentadecanediammonium, dibromide, m.p.=230°C.
Embodiment 3
[0105] Example 3: N,N'-dimethyl-N,N'-diethyl-N,N'-dipropyl-1,16-hexadecanediammonium, dibromide, m.p.=232°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com