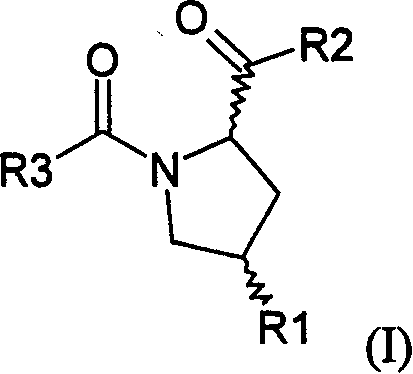
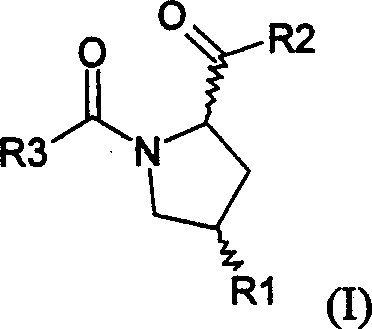
Pyrrolidinyl metalloprotease inhibitor and its application
A technology of methyl pyrrolidine acid and alkyl group, applied in pyrrolidine-based matrix metalloproteinase inhibitors and their application fields, can solve the problems of unsatisfactory performance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: the synthesis of compound of the present invention
[0062] The melting point of the compound was determined by a micro melting point apparatus (the thermometer was not calibrated); thin layer chromatography (TLC, silica gel 60 GF 254 , Qingdao Ocean Chemical Factory) is used to monitor the reaction and check the product purity, using saturated iodine vapor, 10% sulfuric acid ethanol solution, phosphomolybdic acid and other common color developers, 1% FeCl 3 Solution - 2% K 3 Fe(SCN) 6 Solution for detection of phenolic compounds, 1% FeCl 3 The solution is used to detect hydroxamic acid compounds; IR (NICOLET NEXUS470FT-SPECTROMETER type infrared spectrometer is measured, and sample processing adopts potassium bromide tablet method) and ESI-MS (API 4000 type mass spectrometer) by Shandong University School of Pharmacy Determination in the central laboratory; 1 H-NMR was determined by the Institute of Materia Medica, Chinese Academy of Medical Sciences ...
Embodiment 2
[0123] Embodiment 2: The activity of compound of the present invention in vitro and in vivo Inhibition of MMPs test in vitro
[0124] Gelatinase (mmp-2, -9) and TNBS (3,4,5-trinitrobenzenesulfonic acid) were purchased from sigma, and substrates were synthesized as described by Vijaykumar, M.B. et al. Gelatin, substrates and inhibitors were incubated in sodium borate solution (pH 8.5, 50mmol / L) at 37°C for 30min, then added with 0.03% TNBS and incubated for another 20min. The absorbance of the resulting solution was measured at a wavelength of 450 nm.
[0125] Inhibitory activity of compounds of the present invention (IC 50 ) are shown in Table 1 and Table 2.
[0126] Table 1. In vitro inhibitory activity of compound 6-33
[0127]
[0128]
No.
R 1
R 2
R’
4 *
IC 50
(nmol)
6
Oh
OMe
Me
S
657.9±34.6
7
Oh
OMe
Ac
S
nd *
8
OSO 2 CH 3
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com