Medicine composition for improving stability of human urine kininogenase
A technology of urinary kallikrein and its composition, which is applied in the field of pharmaceutical compositions for improving the stability of the biological activity of biochemical drug human urinary kallikrein, and can solve problems such as easy inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
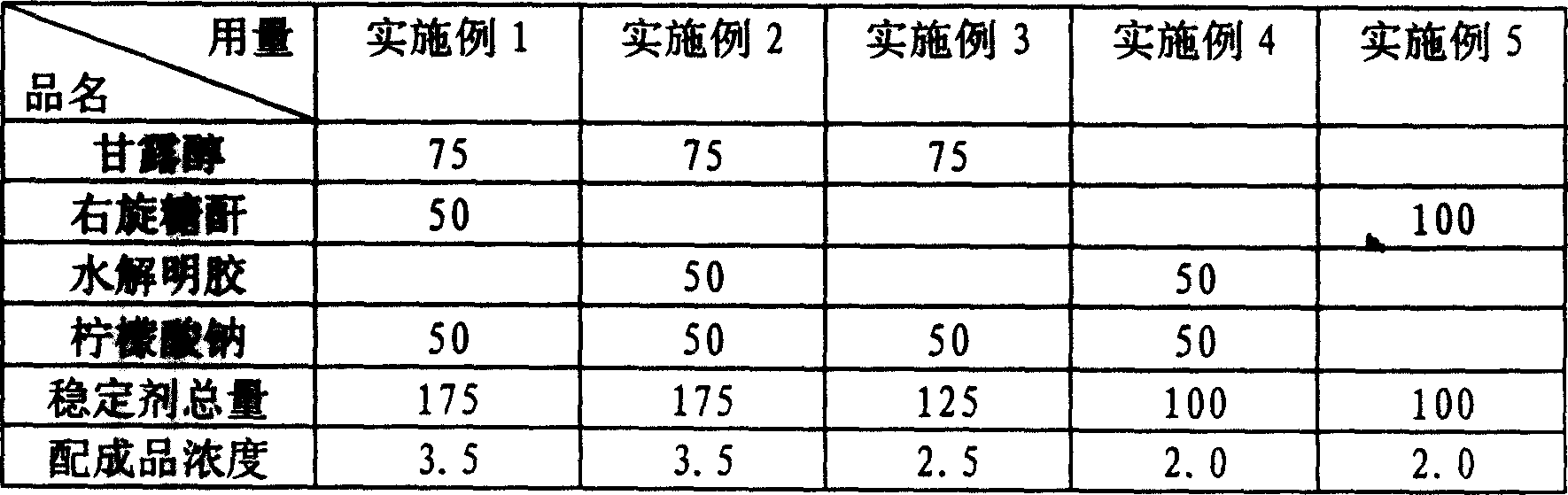
[0014] The quantities listed in the table below were weighed for each stabilizer raw material. The unit of weight is milligrams.
[0015]
[0016] Preparation method: Weigh each medicinal stabilizer according to the species and weight listed in the table, add water for injection to dissolve respectively, adjust the pH value to 6-7 after mixing, then add water for injection to 5000 ml, filter aseptically through filter membrane, and divide Packed in 1,000 ampoules, sealed and sterilized by moist heat.
Embodiment 6
[0017] Embodiment 6 solution stability experiment
[0018] Take one 0.5pNAU human urinary kininogenase freeze-dried powder injection, add one 5 ml of stabilizer solution prepared according to the method of Examples 1 to 5 to dissolve, pour into 100 ml of 0.9% sodium chloride solution and shake well, at 20°C After standing, observe the appearance change at 1, 6, 12, 24, 36, and 48 hours, and take samples to determine the pH value and residual activity. The results are shown in Tables 1-5.
[0019] Take another 0.5pNAU human urinary kininogenase freeze-dried powder injection, dissolve it in 5ml of 0.9% sodium chloride solution, then inject it into 100ml of 0.9% sodium chloride solution, shake well, place it at 20°C, and place it at 1, 6 , 12, 24, 36, and 48 hours to observe the appearance change, take a sample to determine the pH value, and the residual activity as a control, the results are shown in Table 6.
[0020] Sampling time (h)
[0021] Sampling time ...
Embodiment 7
[0027] Embodiment 7 solution stability experiment
[0028] Take one 0.5pNAU human urinary kininogenase freeze-dried powder injection, add one 5 ml stabilizer solution prepared according to the method of Examples 1 to 5 to dissolve, pour into 100 ml of 5% glucose isotonic solution and shake well, at 20°C After standing, observe the appearance change at 1, 6, 12, 24, 36, and 48 hours, and take samples to determine the pH value and residual activity. The results are shown in Tables 7-11.
[0029] Take another 0.5pNAU freeze-dried powder injection of human urinary kininogenase, dissolve it in 5ml 5% glucose solution, then inject it into 100ml 5% glucose solution, shake well, place it at 20°C, and store it at 1, 6, 12, 24 , 36 and 48 hours to observe the appearance change, take samples to determine the pH value, and the residual activity as a control, the results are shown in Table 12.
[0030] Sampling time (h)
[0031] Sampling time (h)
[0032] Samp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com