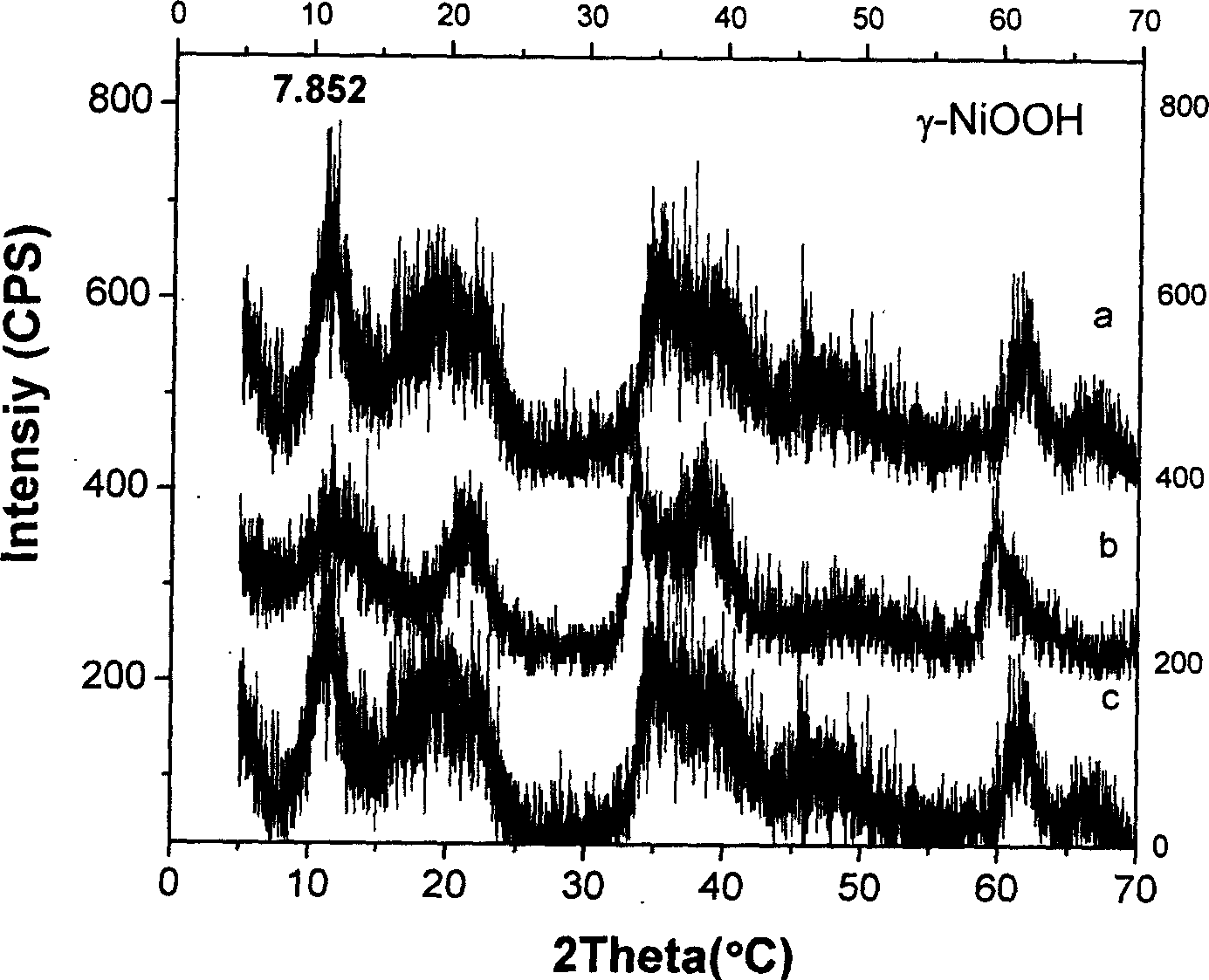
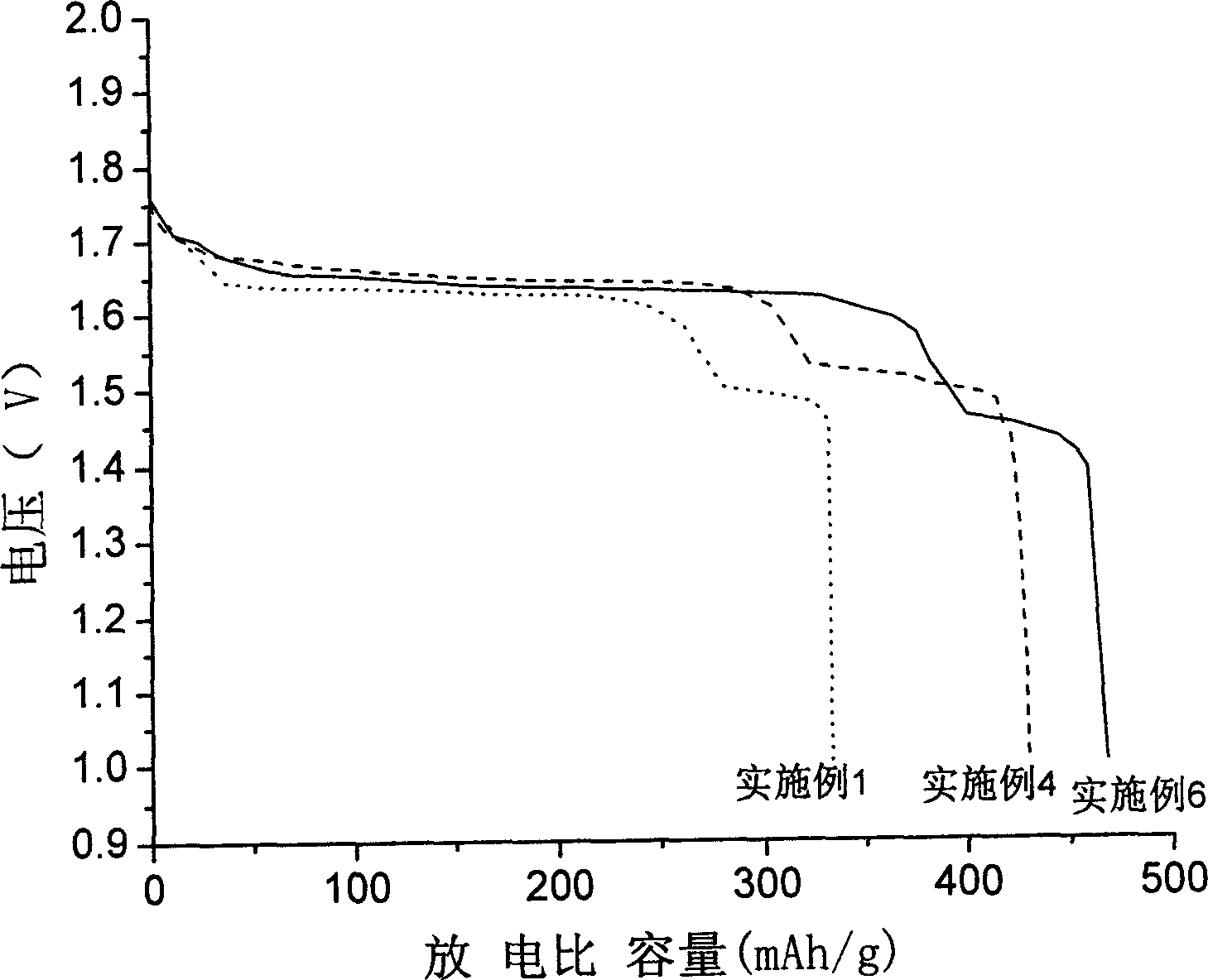
Gamma-hydroxyl nickel oxide and producing process thereof
A technology of nickel oxyhydroxide and oxidant, which is applied in nickel oxide/nickel hydroxide, nickel carbonyl, electrode manufacturing, etc., can solve the problems of battery positive and negative electrode capacity mismatch, waste of power resources, large power consumption, etc., to achieve charging and discharging Stable cycle performance, improved discharge specific capacity, and improved utilization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The first step takes 1.0 moles of nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O) and 0.01 moles (equivalent to 1% of the moles of nickel salt) of aluminum nitrate (Al(NO 3 ) 3 9H 2 0) dissolve with natural water, be mixed with the reaction solution that 100ml nickel ion concentration is 10M;
[0031] In the second step, 0.5 moles (50% of the moles of nickel ions) of potassium peroxodisulfate (K 2 S 2 o 8 ) and 1.2 moles of Potassium Hydroxide, dissolved in 100ml of natural water to make Potassium Hydroxide with a concentration of 12M oxidant solution;
[0032] The third step is to add the reaction solution to the oxidant solution drop by drop under the condition of stirring with the mixer and dispersing with ultrasonic waves at the same time. Disperse for 20 minutes to obtain a black sol-like nickel oxyhydroxide precipitation product;
[0033] The fourth step is to wash the nickel oxyhydroxide with an appropriate amount of water for 5 times, filter it with suction, and c...
Embodiment 2
[0036] The first step takes 0.05 mole of nickel sulfate (NiSO 4 ·6H 2 O), 0.00l moles (equivalent to 2% of the moles of nickel salt) iron nitrate (Fe(NO 3 ) 3 ·7H 2O) and 0.0015 moles (equivalent to 3% of the moles of nickel salt) cadmium chloride (CdCl 2 ), dissolving with distilled water and being mixed with 500ml nickel ion concentration is the reaction solution of 0.1M;
[0037] The second step takes 0.15 moles (equivalent to 300% of the moles of nickel ions) of potassium peroxodisulfate (K 2 S 2 o 8 ), 1.5 moles of potassium hydroxide, dissolved in distilled water to be mixed with 500ml potassium hydroxide concentration is the oxidizing agent solution of 3M;
[0038] The third step is to add the reaction solution dropwise to the oxidant solution under the condition of agitator stirring and ultrasonic dispersion at the same time, control the reaction temperature at 30°C, and the dropwise reaction time is 5 minutes. In 180 minutes, black sol-like nickel oxyhydroxide...
Embodiment 3
[0042] The first step takes 0.1 mole of nickel chloride (NiCl 2 ·6H 2 O), 0.005 moles (equivalent to 5% of the moles of nickel salt) of ferric chloride (FeCl 3 ), was dissolved in deionized water and was mixed with 500ml nickel ion concentration as the reaction solution of 0.2M;
[0043] The second step takes 0.08 moles (equivalent to 80% of the moles of nickel ions) of potassium peroxodisulfate (K 2 S 2 o 8 ), 3.5 moles of sodium hydroxide, dissolved in deionized water to be mixed with 500ml potassium hydroxide concentration is the oxidant solution of 7M;
[0044] The third step is to add the reaction solution to the oxidant solution drop by drop under the condition of agitator stirring and ultrasonic dispersion at the same time, control the reaction temperature to 35°C, and the dropwise reaction time is 10 minutes. In 60 minutes, black sol-like nickel oxyhydroxide precipitation product was obtained;
[0045] The fourth step is to wash the nickel oxyhydroxide with an ap...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com