Macroporous cationic exchanging resin, preparing method and use in synthetic bisphenol A catalyst
A macroporous cation, exchange resin technology, used in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. Poor and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
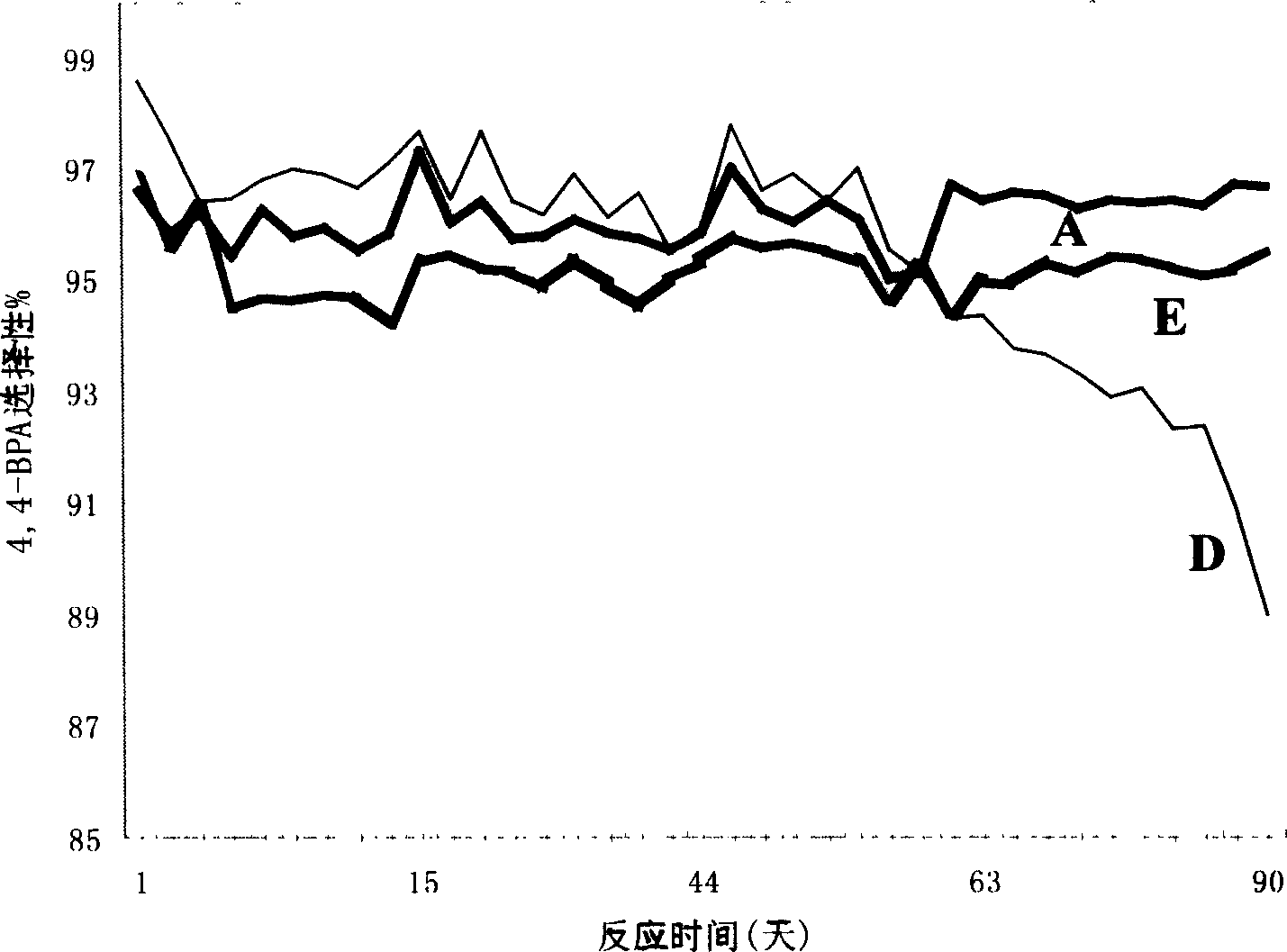
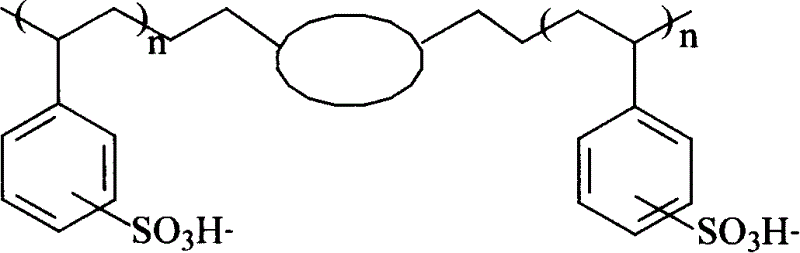
Image
Examples
Embodiment 1
[0033] In a 500 ml four-necked flask equipped with a stirrer, a thermometer, and a condenser, add 1.2 grams of gelatin, 0.2 grams of a dispersing agent, 200 milliliters of water, and 28 grams of sodium chloride. After stirring to dissolve the dispersing agent, add 62.2 grams of Gram styrene, 2.44 gram divinylbenzene (content 52.1%), 60 gram liquid paraffin porogens and 0.65 gram benzoyl peroxide initiator, be warming up to 70 ℃ under stirring, keep warm for 1 hour, then be warming up to 80 ℃ Keep warm for 6 hours, steam the porogen, cool down and discharge, wash with water, dry, and sieve out qualified copolymer white balls.
[0034] In a 1000 ml four-necked flask equipped with a stirrer, a thermometer, and a condenser tube, add 60 grams of the copolymer white balls prepared by the above method, 200 ml of dichloroethane, stir to make the resin swell, and then add 1000 ml of concentrated sulfuric acid , heat up and steam dichloroethane to 100°C, keep warm for 12 hours, cool, fi...
Embodiment 2
[0037] As in Example 1, the consumption of divinylbenzene was changed to 4.88 grams, the liquid paraffin was changed to tert-amyl alcohol, and other conditions were unchanged. The properties of the obtained sulfonated resin (b) and catalyst (B) were shown in Tables 1 and 2.
Embodiment 3
[0039] As in Example 1, 2.44 grams of divinylbenzene was changed to 1.9 grams of divinylphenylmethane, and other conditions were unchanged. The properties of the obtained sulfonated resin (c) and catalyst (C) were shown in Tables 1 and 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com