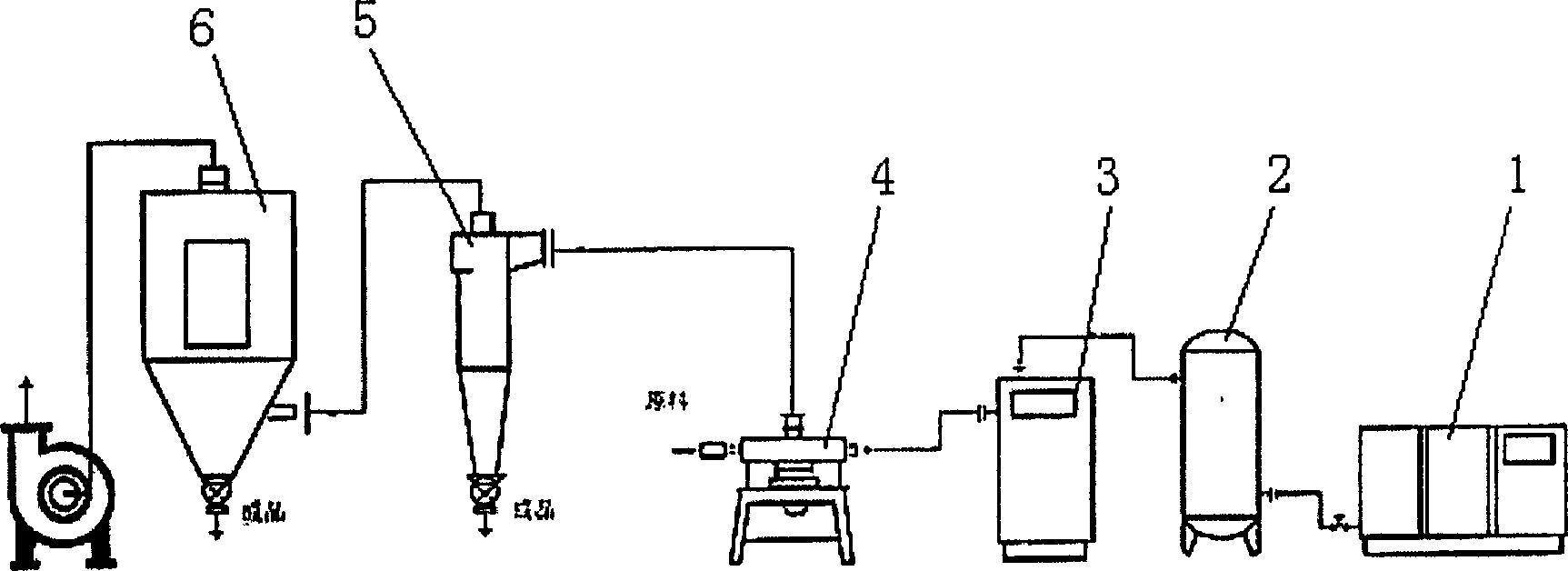
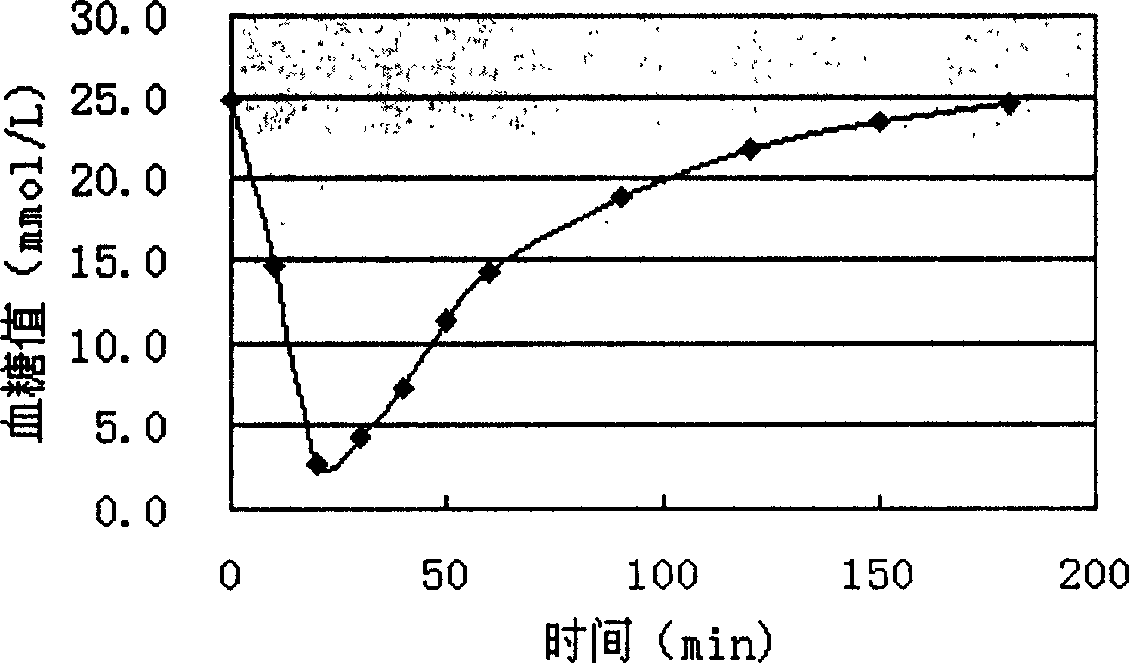
Preparing process of insulin powder inhalant
A technology for dry powder inhalers and insulin, which is applied in the field of preparation of insulin dry powder inhalers, to achieve the effect of reducing the amount of inhalation into the lungs and making the preparation method simple and feasible
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In this embodiment, a commercialized QYN-100 jet mill was used.
[0030] Insulin 20g, leucine 2g, mixed evenly, jet milling feed rate 5g / min, vibration frequency current 55mA, air inlet pressure (impact port pressure) 0.5mpa, feed inlet pressure 0.55mpa, to obtain insulin micronized particles A: Take 200g of mannitol fine powder (passed through a 200 mesh sieve), take 20g of it and leucine 4g and heat and dissolve it with 40ml water to make a soft material, pass through a 40 mesh sieve to granulate, put in an oven at 60°C for about 20 minutes, pass through 60 Mesh sieve for granulation, drying, select powder with 100-360 mesh sieve to obtain carrier B. Mix according to A:B (1:50), pack into capsules to get insulin dry powder inhalation.
Embodiment 2
[0032] In this embodiment, a commercialized QYN-100 jet mill was used.
[0033] After dissolving 2 g of leucine in water, add 20 g of insulin raw material, stir, freeze-dry, and airflow pulverize the freeze-dried powder, feed speed is about 5 g / min, vibration frequency current is 60 mA, air inlet pressure (impact port pressure) is 0.6 mpa. The inlet pressure was 0.7mpa to obtain micronized insulin granules A. The carrier was prepared as in Example 1, mixed according to A:B (1:50), and packed into capsules to obtain the insulin dry powder inhaler.
Embodiment 3
[0035] In this embodiment, a commercialized QYN-100 jet mill was used.
[0036] Add 10 g of insulin raw material, 8 g of mannitol and 0.1 g of poloxamer after dissolving 2 g of leucine in water, stir, freeze-dry, and air-flow pulverize the freeze-dried powder, feed speed is about 5 g / min, vibration frequency current is 60 mA, inlet pressure The impact port pressure) is 0.6mpa, and the feed port pressure is 0.7mpa to obtain insulin micronized particles A. The carrier is prepared as in Example 1, mixed according to A:B (1:50), and packed into capsules to obtain the insulin dry powder inhaler.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com