Medicine for treating hepatitis and its preparing process
A technology for drugs and hepatitis, applied in the field of drugs and their preparation, can solve the problems of drugs affecting the safety of clinical medication, product quality and stability, failing to reflect the purity of glycyrrhizic acid and its salts, and restricting the scope of use, so as to reduce toxic and side effects. and stimulatory effect, less side effects, reducing the effect of stimuli
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A medicine for treating hepatitis, characterized in that the active ingredient is a compound represented by the following structural formula (I) with a liquid chromatography purity of 98%, wherein R1, R2, and R3 are H + and / or NH 4 + .
[0028]
[0029] The preparation method of above-mentioned pharmaceutical active ingredient (I) described in the present invention comprises following processing steps:
[0030] A, get the crude product of glycyrrhizic acid or its salt, dissolve with mobile phase, get crude product solution;
[0031] B, filter the crude product solution with a 0.22 μ microporous filter membrane, then inject the filtrate into a chromatographic column with octadecylsilane bonded silica gel (C18) as a filler, and select aqueous acetic acid and acetonitrile by high performance liquid chromatography The mixed solution is used as the mobile phase, and 250nm is selected as the detection wavelength, and the crude product solution of glycyrrhizic acid or it...
Embodiment 2
[0038] Embodiment 2: the preparation of high-purity (HPLC) glycyrrhizic acid
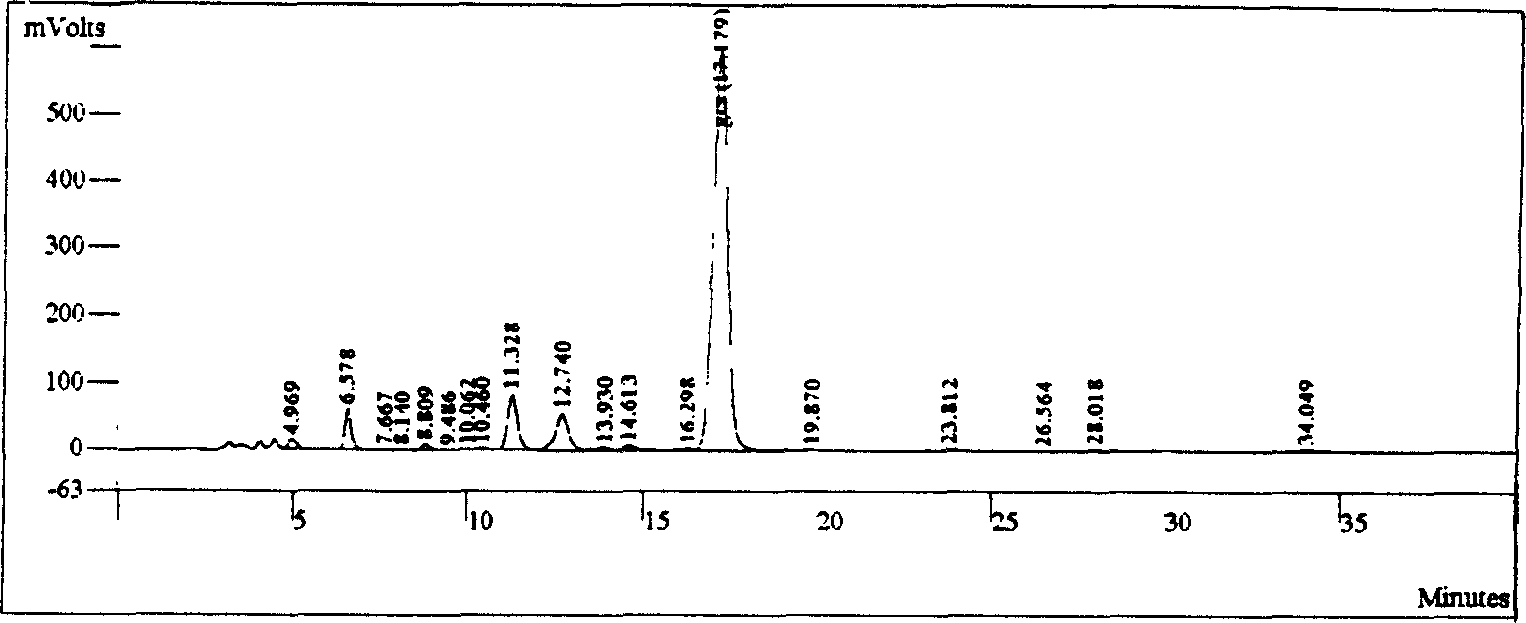
[0039] Take 200 g of crude glycyrrhizic acid, dissolve it in 3.5 L of mobile phase, and filter it with a 0.45 μ microporous membrane. Get glycyrrhizic acid crude product solution 100ml and inject in chromatograph, chromatographic condition: chromatographic column is Varian C18 10 μ (41.4 * 250mm), and mobile phase is 10% acetic acid aqueous solution-% acetonitrile (70: 30), and detection wavelength is 252nm, and flow velocity is 40ml / min. The component solutions were collected and evaporated to dryness under reduced pressure in a 40° C. water bath with a rotary evaporator to obtain 129.6 g of white solid powder with a yield of 64.8%.
[0040] HPLC purity
Embodiment 3
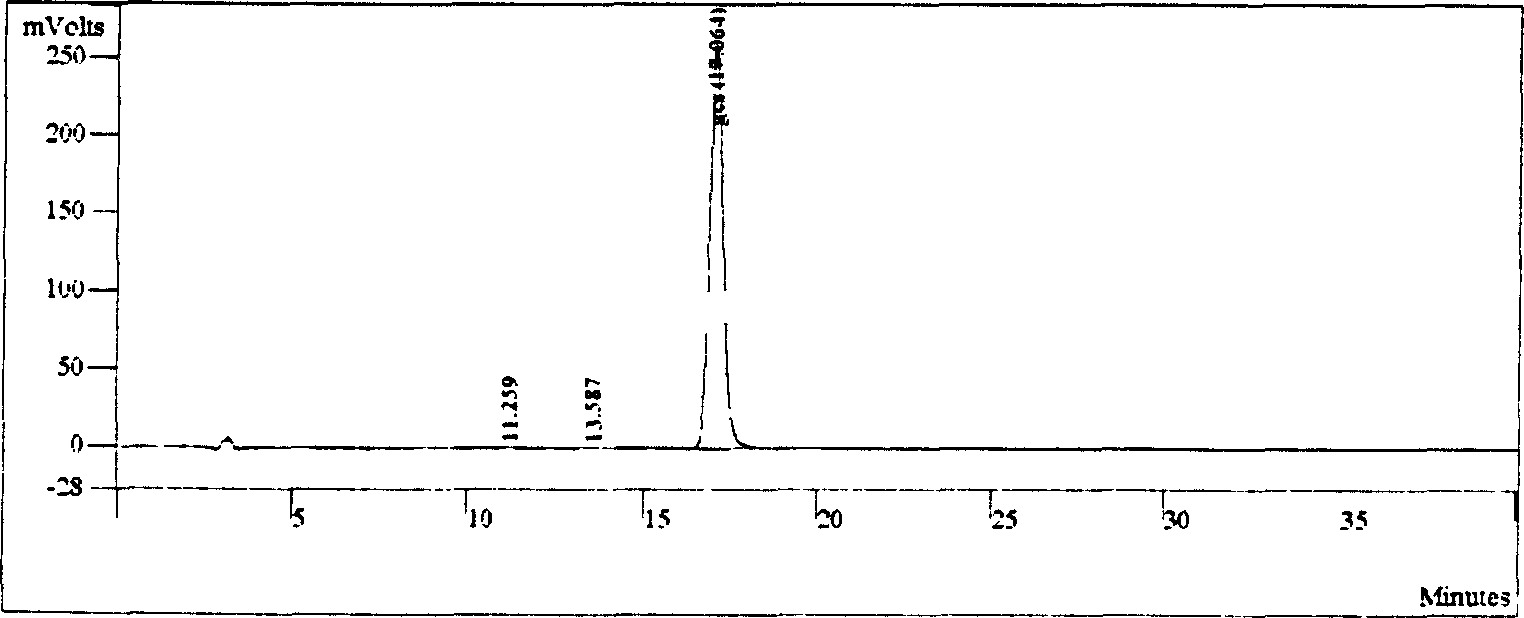
[0041] Embodiment 3: the preparation of diammonium glycyrrhizinate (or monoammonium glycyrrhizinate)
[0042] HPLC purity
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com