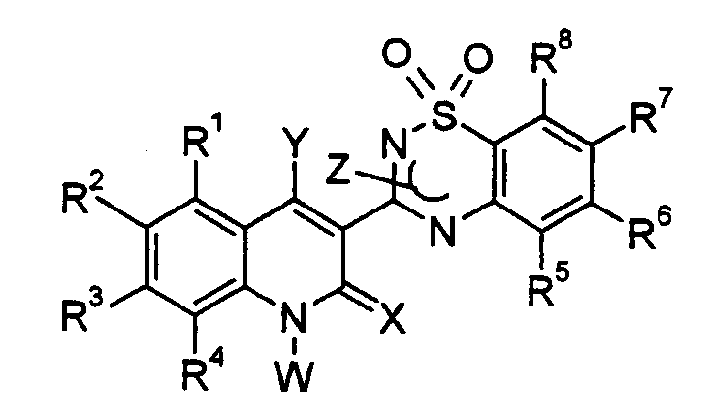
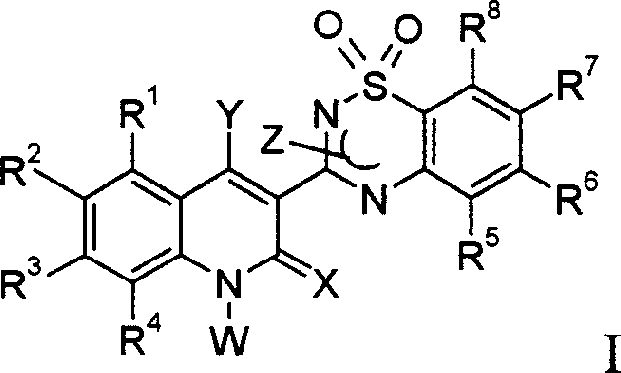
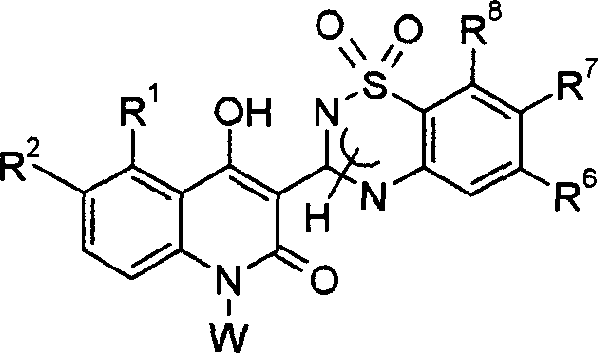
Novel anti-infectives
A compound, alkyl technology, applied in the field of hepatitis C virus inhibitors, can solve the problem of weakened response in genotype 1 patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0143] Alternative methods for the preparation of N-alkylated 1H-benzo[d][1,3]oxazine-2,4-diones (d) are shown in Scheme 5 and Scheme 6.
[0144] Option 5
[0145]
[0146] Conditions: i.W-CHO, NaBH 4 .THF; ii. Triphosgene, THF
[0147] One of the alternative methods for the preparation of N-alkylated 1H-benzo[d][1,3]oxazine-2,4-diones (d) is shown in Scheme 5, involving the reductive amination conditions 2-Aminobenzoic acid (a) is treated with the appropriate aldehyde ( W-CHO) treats 2-aminobenzoic acid to generate N-alkylated 2-aminobenzoic acid (r). Treatment of the N-alkylated 2-aminobenzoic acid (r) with phosgene or a phosgene equivalent such as triphosgene or ethyl chloroformate in a suitable solvent such as tetrahydrofuran as described above affords the N-alkylated 1H-Benzo[d][1,3]oxazine-2,4-dione (d).
[0148] Option 6
[0149]
[0150] Condition: i.W-NH 2 , 6mol% CuBr 2 , K 2 CO 3 , THF, 63°C; ii. Triphosgene, THF
[0151] Scheme 6 illustrates an...
test approach 1
[0157] Method for detecting positive strand replicon HCV-RNA in replicon cells
[0158] at 37°C and 5% CO 2 The replicon cells in DMEM (Dulbecco's Minimal Essential Medium) were mixed at 3×10 per well 3 The cells were plated in 96-well plates in DMEM containing 10% FCS (fetal calf serum), 1% NEAA (non-essential amino acids) and 1 mg / ml Geneticin (G418 neomycin). After allowing the cells to attach for 4 hours, 1 μl of the candidate antiviral agent solution was added to the medium (n=8 wells / dilution). Briefly, eleven 2.5-fold serial dilutions of test compounds from 1 mM stocks in DMSO (dimethyl sulfoxide) were prepared at final concentrations ranging from 10000 to 1.0 nM. Plates were incubated for 40 hours until reaching 80% confluency. After the medium was removed, 150 μl Buffer RLT (Qiagen, Valencia, California, US) was added to each well, and the RNA was purified according to the manufacturer’s recommendation (Qiagen RNAeasy), and 45 μl of dH 2 Dilute twice in O. Add ap...
test approach 2
[0160] Method for detecting negative strand replicon HCV-RNA in replicon cells
[0161] For strand-specific detection, perform a reverse transcription (RT) reaction using primers containing HCV RNA (or a replicon RNA sequence such as the neomycin gene) and an 18-base tag at the 5′ end of an unrelated sequence , 5'ACATGCGCGGCATCTAGACCGGCTACCTGCCCATTC3' (SEQ ID NO 4). RT reaction was carried out with Thermoscript-RT-PCR system (Invitrogen) according to the instruction manual, about 9 μl of RNA was harvested from the cells, and it was incubated with 1 μl of primer (10 μM) at 60° C. for 1 hour at RT. Afterwards, 2 μl of the cDNA product containing the 5′ tag was amplified for TaqMan quantification using 48 μl of TaqMan Universal Master Mix (Applied Biosystems) and the following primers: neo-forward tag: 5′ACA TGC GCG GCA TCT AGA 3' (SEQ ID NO 3); neo reverse: 5' CCAGATCATCCTGATCGACAAG 3' (SEQ ID NO 6); neo probe: 5' FAM-ACA TCG CAT CGA GCG AGC ACG TAC-TAMRA 3' (SEQ ID NO 3). The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com