Lithium battery
A lithium battery, non-aqueous electrolyte technology, applied in secondary batteries, battery electrodes, non-aqueous electrolyte batteries, etc., can solve the problem that the charging and discharging characteristics of lithium batteries cannot be fully improved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
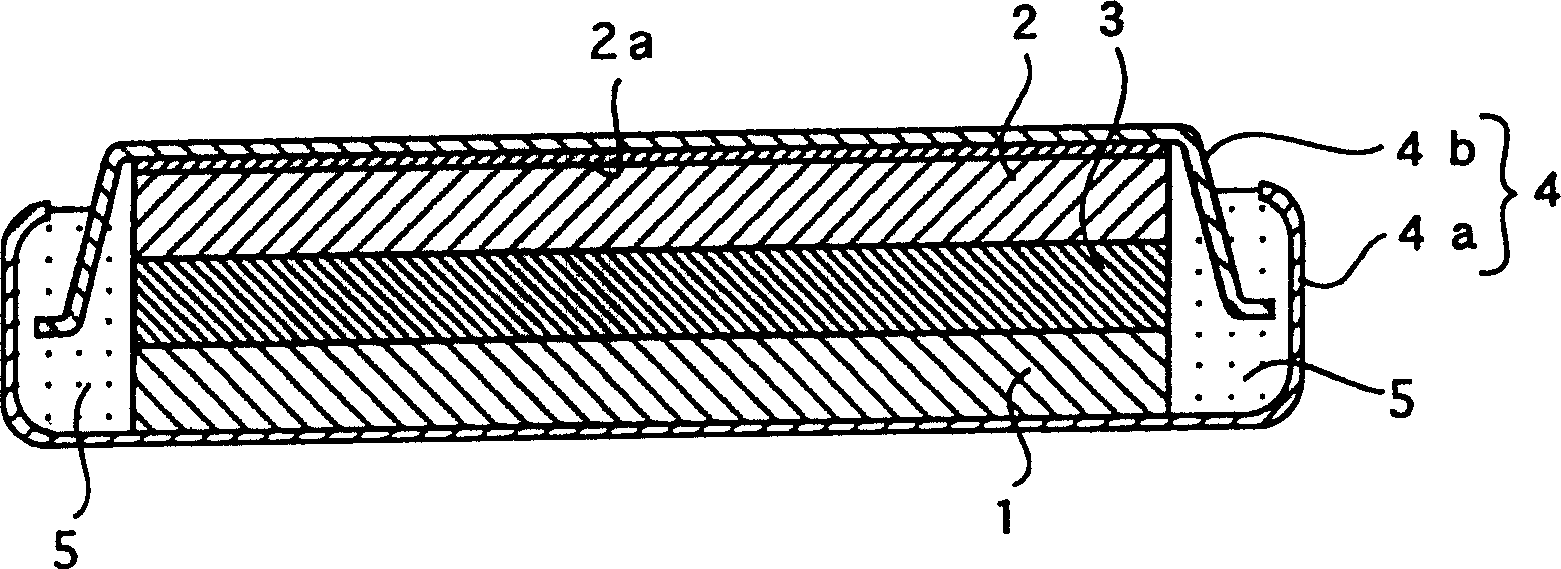
[0045] In embodiment 1, made diameter 24.0mm, thickness 3.0mm such as figure 1 A flat coin-type laboratory battery is shown.
[0046] In order to make the working electrode for forming the negative electrode of this experimental battery, a carbon material made as follows was used, that is, after making graphite powder (d 002 = 0.336nm, L C >100nm) is immersed in molten pitch, and the surface of the graphite powder is covered with pitch by drying it, and the graphite covered with pitch is fired at 1100° C. in a nitrogen atmosphere for 2 hours, so that the graphite The surface is covered with low crystalline carbon. Furthermore, when the surface of the graphite powder was covered with pitch as described above, in this Example 1, the coating amount of the pitch was 8 parts by weight with respect to 100 parts by weight of the graphite powder.
[0047] In addition, the above-mentioned carbon material was irradiated with an argon ion laser with a wavelength of 514.5 nm using a Ra...
Embodiment 2~6 and comparative example 1、2
[0055] In embodiment 2~6 and comparative example 1,2, the amount of vinyl vinyl carbonate (VEC) and ethylene carbonate (VC) added in the non-aqueous electrolytic solution of described embodiment 1 is changed, Except for this, in the same manner as in the case of the above-mentioned Example 1, each experimental battery of Examples 2 to 6 and Comparative Examples 1 and 2 was produced.
[0056] Here, the amounts of vinyl vinyl carbonate (VEC) and ethylene carbonate (VC) added to 100 parts by weight of the non-aqueous electrolytic solution were changed to, in Example 2, 10 parts by weight of vinyl vinyl carbonate Ester (VEC), 2 parts by weight ethylene carbonate (VC), in embodiment 3 is 5 parts by weight vinyl vinyl carbonate (VEC), 4 parts by weight ethylene carbonate (VC), in embodiment 4 is 10 Parts by weight of ethylene carbonate (VEC), 4 parts by weight of ethylene carbonate (VC), are 5 parts by weight of ethylene carbonate (VEC) in embodiment 5, do not add ethylene carbonate...
Embodiment 7
[0066] In embodiment 7, in making the working pole of the negative electrode of described embodiment 1, use the same graphite powder as described embodiment 1 (d 002 = 0.336nm, L C >100nm), after this graphite powder is immersed in the pitch of molten state, when by making it dry and cover the surface of graphite powder with pitch, make relative to 100 weight parts of graphite powder, the covering amount of pitch reaches 5 parts by weight, Except for this, in the same manner as in the case of the above-mentioned Example 1, a carbon material was produced, and at the same time, a working electrode to be a negative electrode was produced.
[0067] Here, for this carbon material, the R value (I D / I G ) and R A value (I A / I G ), the results are shown in the following table 2, the R value (I D / I G ) is 0.31, R A value (I A / I G ) is 0.12.
[0068] In addition, except for using the working pole, as in the case of the above-mentioned Example 3, 5 parts by weight of ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com