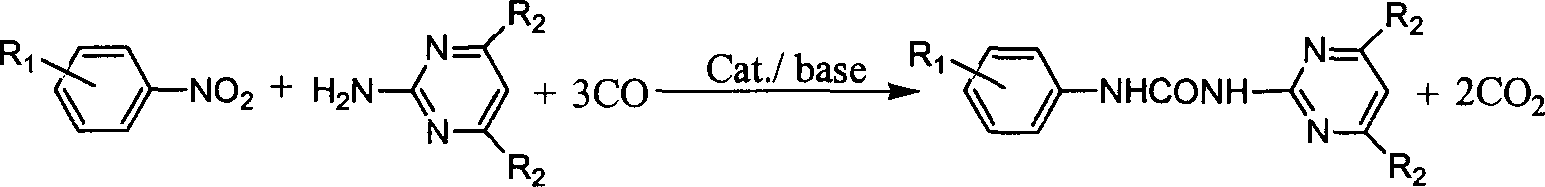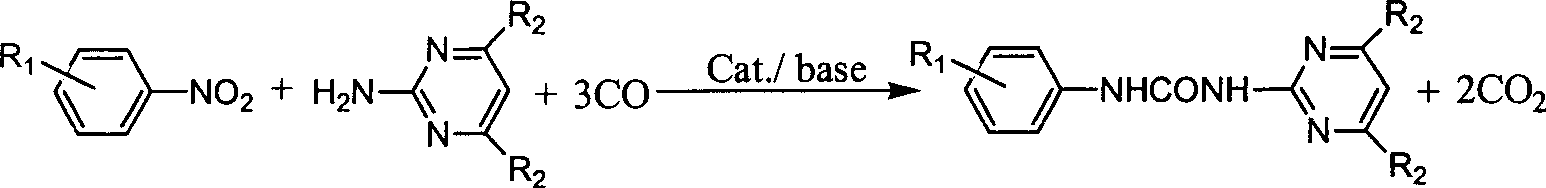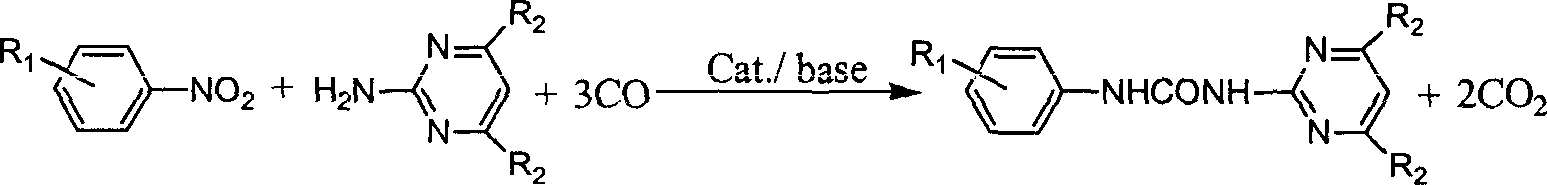N-phenyl-N'-pyrimidinyl-substituted urea derivative synthesizing method
A synthesis method and derivative technology, which is applied in the field of synthesis of N-phenyl-N'-pyrimidinyl substituted urea derivatives, can solve the problem of corrosive by-products containing large amounts of chlorine, the total yield is not too high, and it is easy to pollute the environment and other problems, to achieve the effect of cheap catalyst, less investment in equipment, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1 N-phenyl-N'-(2-pyrimidinyl)urea
[0017] Add nitrobenzene (10mmol), selenium dioxide (0.5mmol), 2-aminopyrimidine (10mmol), triethylamine (20mmol) and toluene 10ml in a 100ml stainless steel autoclave, and raise the pressure of CO after three times of replacement with CO to 3.0 MPa, put it into an oil bath at 150° C. and stir for 4 hours, cool to room temperature, filter the reaction product, and purify the filtered crystals through column chromatography. The eluent is petroleum ether: ethyl acetate (10 : 3), concentrated eluate to obtain product N-phenyl-N'-(2-pyrimidinyl)urea, melting point 233~235°C, yield 79.8%.
Embodiment 2
[0018] Example 2 Synthesis of N-phenyl-N'-(2-pyrimidinyl) urea derivatives
[0019] Present embodiment is summarized as follows in tabular form: (its reaction condition and step are with embodiment 1)
[0020]
[0021] Table 1: Synthesis of N-phenyl-N'-(2-pyrimidinyl)urea by carbonylation of substituted nitrobenzene and 2-aminopyrimidine
[0022] Derivatives
[0023] product
[0024] Serial number R 1 R 2
[0025] Yield(%)
[0026] Melting point (°C)
[0027] 1 H H 233~235 79.8
[0028] 2 2-Me H 225 71.3
[0029] 3 3-Me H 194~195 77.2
[0030] 4 4-Me H 211~213 74.9
[0031] 5 2-Cl H 238~239 53.4
[0032] 6 3-Cl H 226~227 88.7
[0033] 7 4-Cl H 241~243 27.1
[0034] 8 2-iPr H 193~194 35.0
[0035] 9 3-iPr H 202~203 88.1
[0036] 10 4-iPr H 268~269 68.6
[0037] 11 3-COCH 3 H 229~231 77.3
[0038] 12 4-COCH 3 H 252~255 63.7
[0039] 13-CF 3 ...
Embodiment 3
[0046] Example 3 Synthesis of N-phenyl-N'-(4,6-dimethoxy-2-pyrimidinyl)urea derivatives, the reaction conditions and steps are the same as in Example 1, and the results are listed in Table 2:
[0047] Table 2: Synthesis of N-phenyl-N'-(4,6-dimethoxypyrimidinyl) urea derivatives by carbonylation of substituted nitrobenzene and 2-amino-4,6-dimethoxypyrimidine
[0048] product
[0049] Serial number R 1 R 2
[0050] Yield(%)
[0051] Melting point (°C)
[0052] 20H OMe 214~216 77.1
[0053] 21 2-Me OMe 205~207 69.9
[0054] 22 3-Me OMe 223~224 71.0
[0055] 23 4-Me OMe 248~249 70.5
[0056] 24 2-Cl OMe 195~197 43.9
[0057] 25 3-Cl OMe 227~230 86.6
[0058] 26 4-Cl OMe 246~247 23.9
[0059] 27 2-iPr OMe 164~166 36.6
[0060] 28 3-iPr OMe 166~167 83.9
[0061] 29 4-iPr OMe 216~217 66.3
[0062] 30 3-COCH 3 OMe 216~218 74.9
[0063] 31 4-C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com