Modified lithium-cobalt oxide used for positive pole of lithium ion cell, its prepn and lithium ion cell
A lithium cobalt oxide, lithium ion battery technology, applied in lithium compounds, battery electrodes, chemical instruments and methods, etc., can solve the problems of decreased yield, unfavorable charging potential of lithium ion batteries, complicated preparation by hydrothermal oxidation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: LiCoO 2 -ZrO 2
[0023] LiCoO with a particle size distribution of 90% particle size less than 17 microns 2 1.9 g of pellets were impregnated with 0.1 g of ZrO(NO 3 ) 2 .xH 2 O (molecular weight 231.23, ALDRICH, USA, code number 34646-2) in 20 ml of aqueous solution, the resulting mixture was dried at 110°C, and the obtained dry mixture was calcined at 600°C for 3 hours. The calcined product can be used as the positive electrode material of the lithium ion battery after grinding.
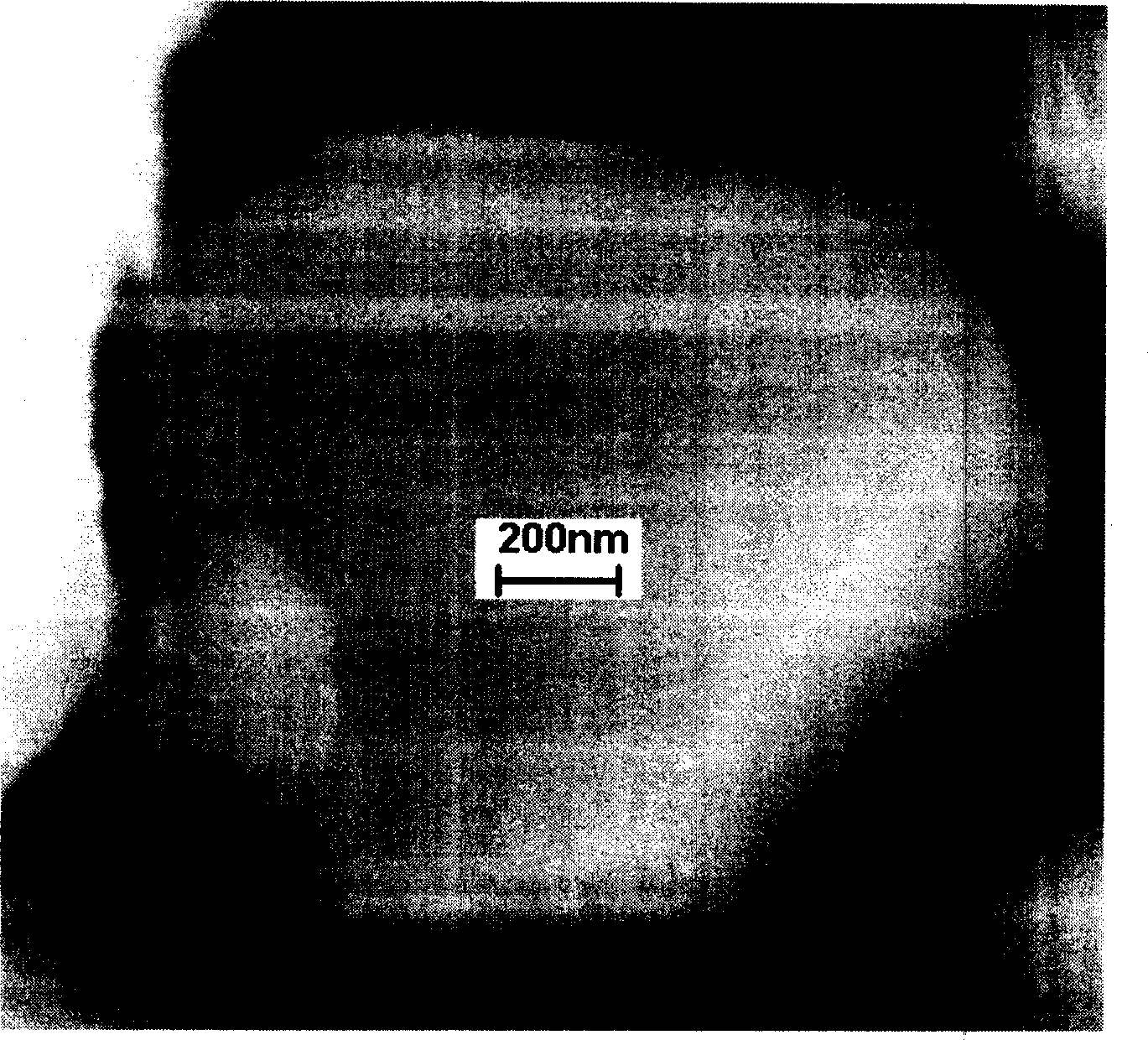
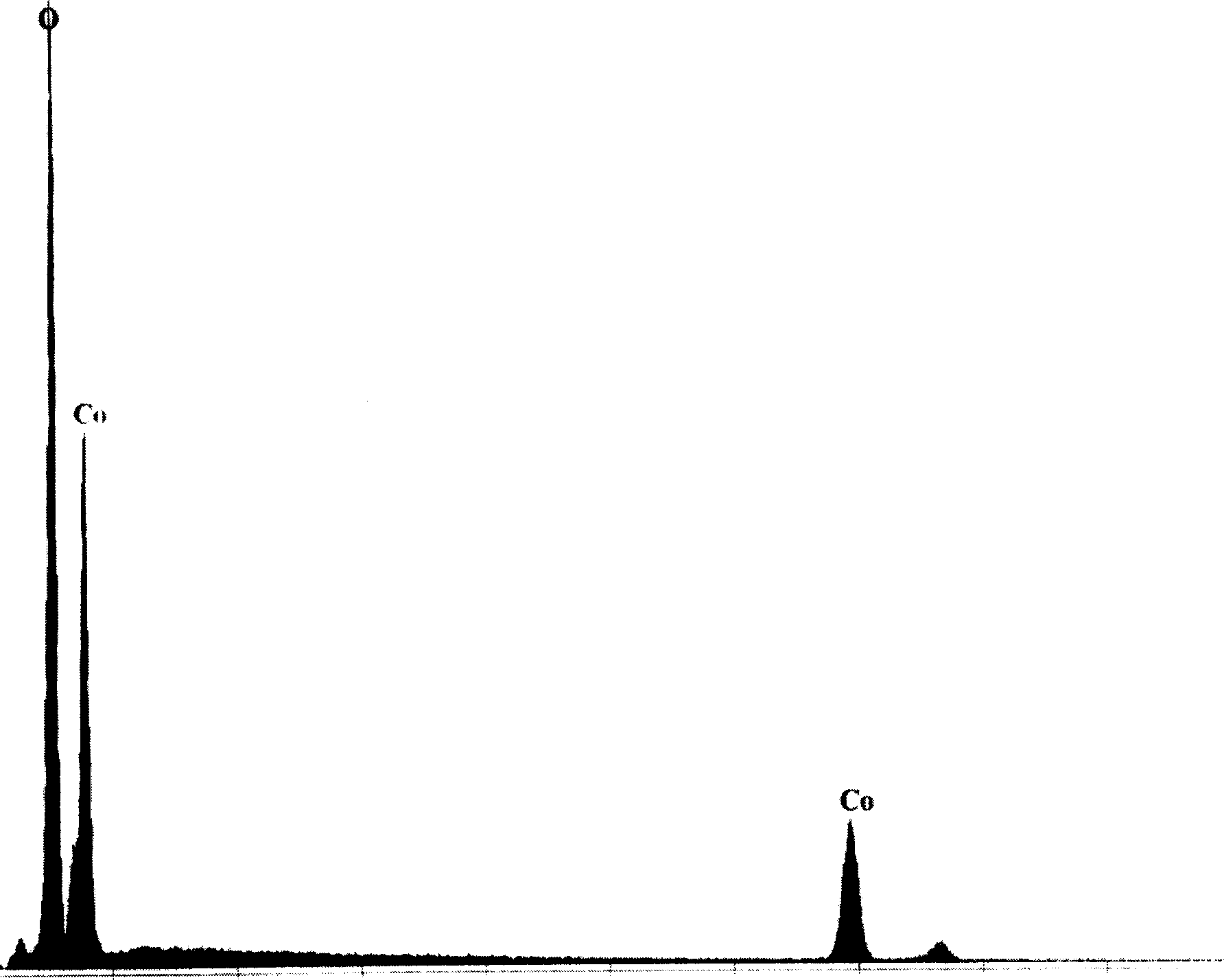
[0024] figure 1 Shown is LiCoO before modification 2 SEM photographs of the particles, while figure 2 is its EDS spectrum. from figure 1 It can be seen that LiCoO 2 The surface of the particles is quite smooth, while figure 2 Then peaks of oxygen and Co are displayed.
[0025] Modified LiCoO 2 The SEM photographs are shown in image 3 , from which it can be seen that LiCoO 2 The particles have substances attached to their surfaces. Figure 4 For the modified LiCo...
Embodiment 2
[0026] Example 2: LiCoO 2 -B 2 o 3
[0027] In addition to 0.1 g of boric acid (H 3 BO 3 , molecular weight 61.83) powder (ALDRICH, the United States, code 23646-2) to replace 0.1 g of ZrO (NO 3 ) 2 .xH 2 O outside, repeat the step of embodiment 1.
[0028] Using Examples 1-2 and commercial LiCoO 2 (Nippon chemical Industrial Co., Japan, code-named CELLSEED C), making the positive electrode, using lithium metal as the negative electrode, and 1M LiPF with a mixed solvent of EC (ethylene carbonate) and DEC (diethyl carbonate) 6 The solution was used as an electrolyte to assemble a 2030-Type button half-cell. Tested at a charge and discharge rate of 0.2C (current density 28mA / g). When commodity LiCoO 2 When the battery with the material as the positive electrode is charged and discharged at a high potential (3-4.4V), although the charge-discharge at a lower potential (3-4.2V) can increase the battery life by about 20%, it is found that the long-term cycle test is as fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com