Method for production of stroma-free hemoglobin
A technology of hemoglobin and blood, applied in the system field, can solve problems such as pollution and high cost, and achieve the effect of low-cost collection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
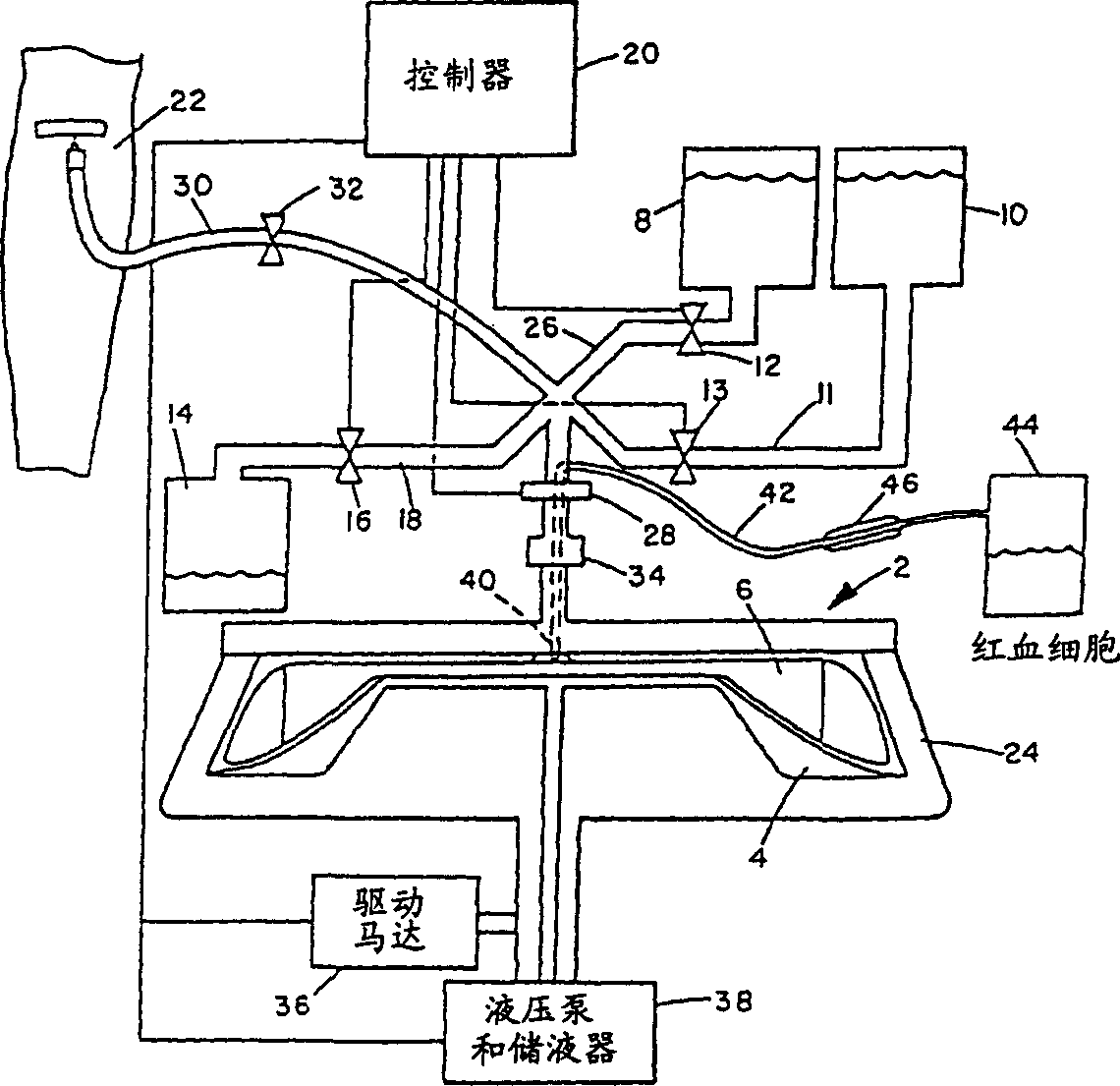
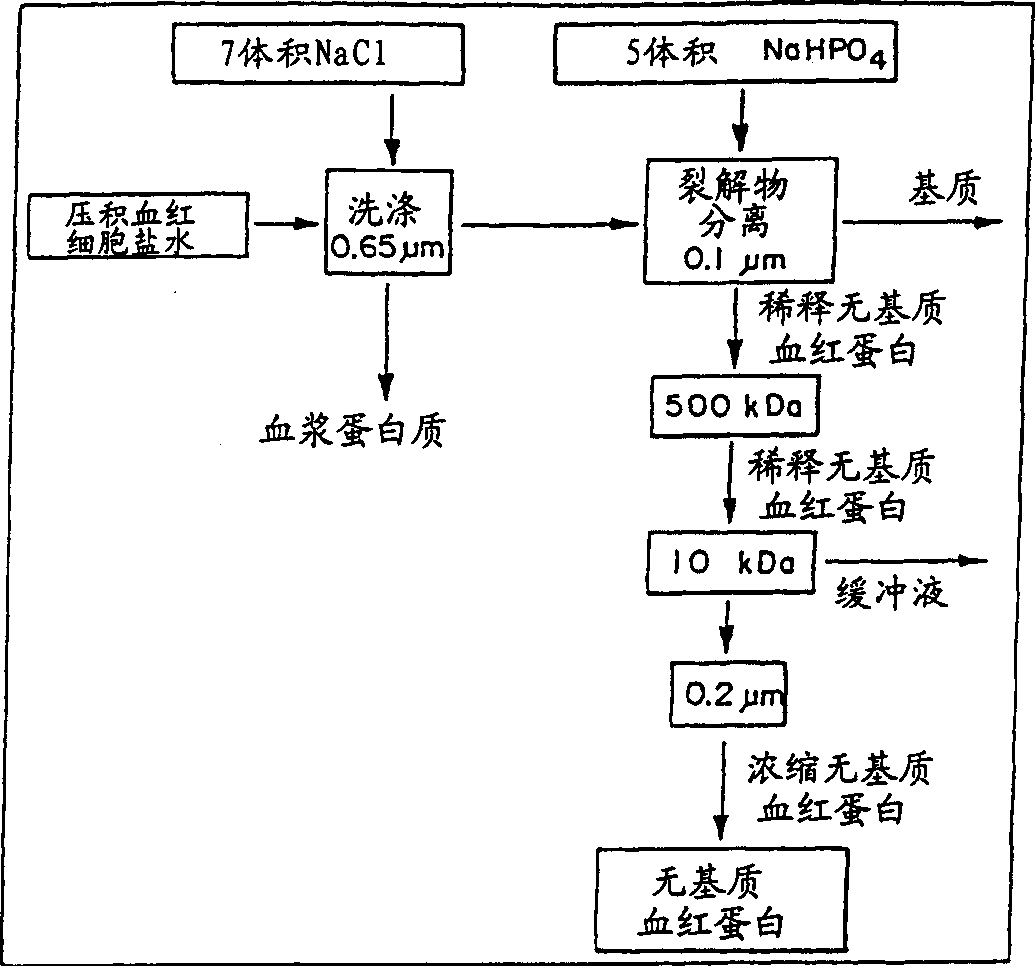
[0028] An experimental apparatus was constructed for the production of stroma-free hemoglobin from blood, which included a collection of emptied bags for expired blood; a cross-filter unit for washing, lysing, and purification; a bioreactor for cross-linking hemoglobin; preparative-grade high-efficiency liquid a phase chromatography (HPLC) instrument; and a fume hood where the product is finally filled into sterile bags. The flow circuits, including the PFW dispersion circuit, are sealed. All fittings are sanitary three-clamp (through) type, manufactured in a laminar flow environment. Excluding the office, the complete process takes about 1000ft 2 laboratory space. The capacity of the pilot plant was about 5 liters per week and the product cost was about $1000 / liter.
[0029] The cross filtration system consists of three pumps, four tanks and 4 different filter components. All construction materials are constructed of 316L stainless steel with an internal finish of 180 gri...
Embodiment 2
[0040] In an embodiment of the present invention, a self-contained portable device designed to collect donor / patient blood at the bedside is used. Suitable systems for this use include the Haemonetics MCS+8150 Blood Collection System (Haemonetics Corporation, Braintree, MA) and the COBE 2991 Cell Processor (COBE Laboratories, Inc., Lakewood, CO). Blood was collected in a CP2D / AS-3 anticoagulant / supplement system with a ratio of anticoagulant to anticoagulated blood of 1:16. The machine is configured to collect one or two units of red blood cells from a donor in about 25 minutes. Each unit collected yielded approximately 180 ml packed red blood cells ("RBC") and 400 ml plasma. The efficiency of the separation can be determined using the following relationship:
[0041] RBC count after treatment × weight after treatment × 100 = % RBC recovery
[0042] RBC count before treatment × weight before treatment
[0043] The number of lymphocytes remaining in the packed red blood cel...
Embodiment 3
[0067] Example 3: Preparation of modified hemoglobin using the described invention
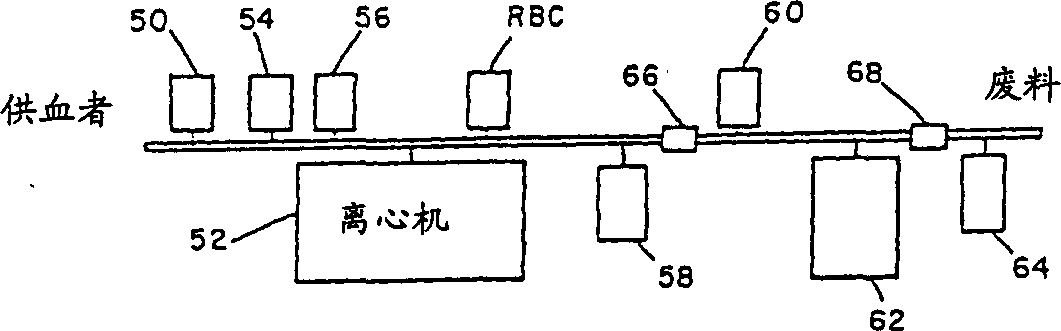
[0068] The present invention can also be used in Figure 4 A modified hemoglobin solution ("product") is prepared from the stroma-free hemoglobin (SFH) solution prepared in the modified cell separator device shown.
[0069] Red blood cells can be obtained from any source, including animal or human, stored or fresh, or even expired human (blood) units obtained from blood banks. The improved procedure can also be used with hemoglobin solutions (including recombinant hemoglobin) prepared by any method, including the present invention or other methods. If donor blood is used, it is first mixed with an anticoagulant 50 , washed with saline 54 in a centrifuge rotor 52 , and lysed with distilled water 56 . The plasma, platelet and white blood cell fractions are removed and stored in separate compartments 58. A solution of hemoglobin (SFH) 60 is placed in a separate compartment reservoir 62 or cent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com