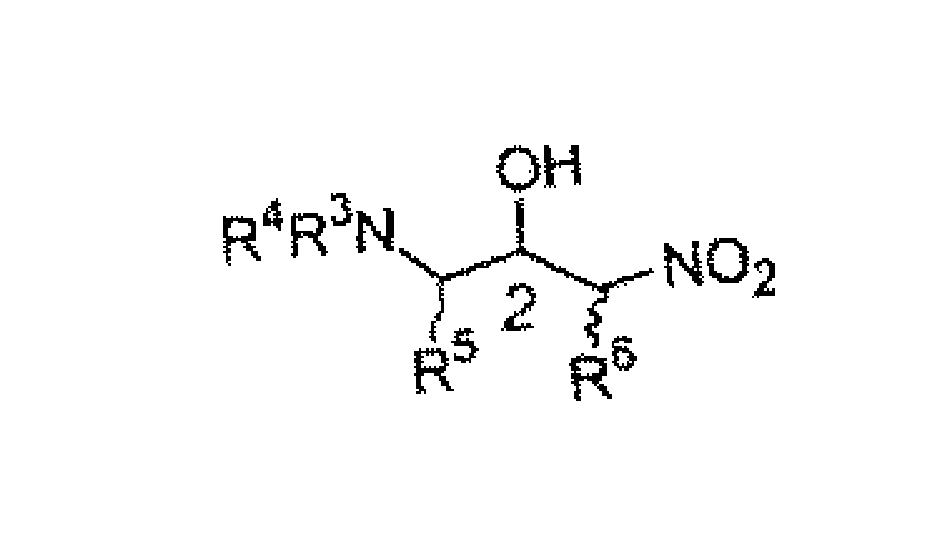
Chiral amine compound and its synthesis and use
A synthesis method and technology of chiral amines, applied in the field of chiral amine compounds, can solve problems such as narrow substrate range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Reaction 4
[0026] Dissolve 50 mg of N,N-dibenzyl-(S)-phenylalaninaldehyde 5 (0.15 mmol) in 1.5 mL of toluene, then add 6 mg of 1,3-N,N-di-(R)-α-naphthalene Etamine 8, after cooling to -20°C, 0.2 mL of nitromethane was added dropwise, reacted for 12 hours, and 55 mg of the post-treated product was obtained, with a total yield of 92%. d.e. >99%. 1 HNMR (300MHz, CDCL 3 )δ compound 6: 7.32 (m, 15H), 4.85 (dd, 1H, J 1 =12.0Hz,J 2 =3.0Hz), 4.53(m, 1H), 4.06(dd, 1H, J 1 =13.5Hz,J 2 =9.9Hz), 3.84(d, 2H, J=13.5Hz), 3.57(d, 2H, J=13.5Hz), 3.20(dd, 1H, J 1 =6.0Hz,J 2 =3.3Hz), 3.08(m, 2H), 2.53(s, 1H).ESIMS: (M+H) + 391.3. Compound 7: 7.32 (m, 15H), 4.19-4.33 (m, 2H), 4.13 (d, 2H, J=13.2Hz), 3.95 (s, 1H), 3.83 (d, 1H, J=21.3Hz), 3.49(d, 2H, J=13.2Hz), 3.24(d, 1H, J=11.4Hz), 2.86(m, 2H); ESIMS: (M+H) + 391.3.
Embodiment 2
[0028] Dissolve 50mg of N,N-dibenzyl-(S)-phenylalaninaldehyde 5 (0.15mmol) in 1.5mL of toluene, then add 6mg of guanidine 9, cool to -20°C and add 0.2mL of nitromethane dropwise, After 12 hours of reaction, 55 mg of product was obtained after post-treatment, with a total yield of 89%. d.e. = 93.3%. The structural data are the same as in Example 1.
Embodiment 3
[0030] Dissolve 50mg of N,N-dibenzyl-(S)-phenylalaninaldehyde 5 (0.15mmol) in 1.5mL of toluene, then add 6mg of guanidine 10, cool to -20C, and drop 0.2mL of nitromethane into the reaction After 12 hours, 55 mg of the product was obtained after post-treatment, with a yield of 97%. d.e. = 93.3%. The structural data are the same as in Example 1. Example 4
[0031] Dissolve 50mg of N,N-dibenzyl-(S)-phenylalaninaldehyde 5 (0.15mmol) in 1.5mL of toluene, then add 6mg of guanidine 11, cool to -20°C and add 0.2mL of nitromethane dropwise, After 18 hours of reaction, 55 mg of product was obtained after post-treatment, with a total yield of 91%. d.e. = 57.4%. The structural data are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com