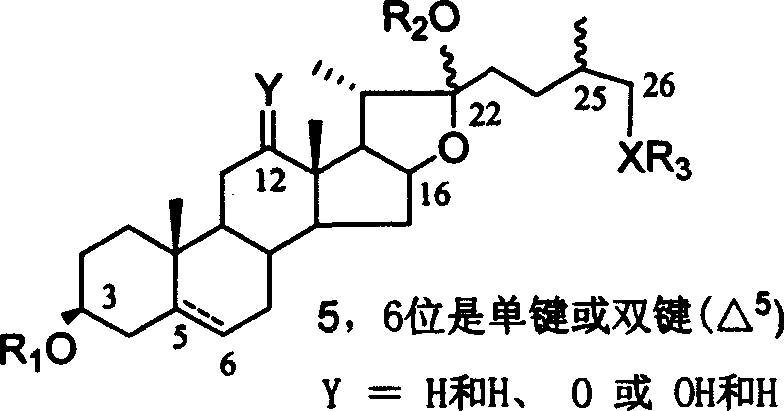
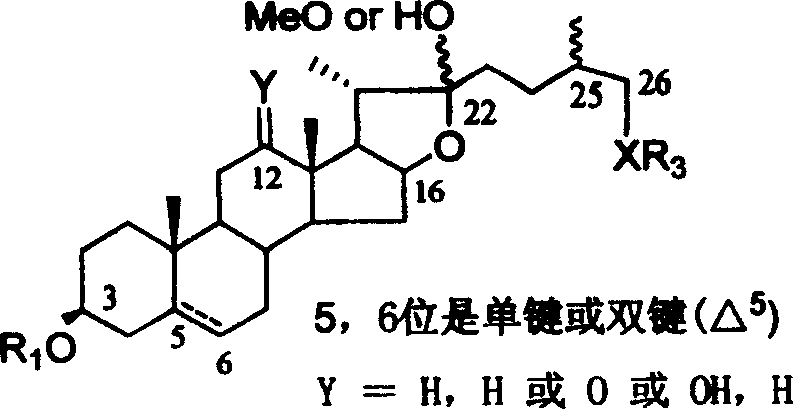
Chemical synthesis method of franosterol saponin and its derivative
A technology of furostanol saponins and a chemical method is applied in the field of chemical synthesis of furostanol saponins and derivatives thereof, and can solve the problems of limiting compound structure-activity relationship, difficulty in separation and purification and partial degradation, and restricting development and utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0046] The following examples will help to understand the present invention, but do not limit the content of the present invention.
[0047] Synthetic example
[0048] Synthesis of 26-thiofurostanol saponin VIII:
[0049]
[0050] Reaction scheme 1: Synthesis of 26-position thiofurostanol saponin VIII
[0051] Reagents and conditions: a) 4 Å MS, trimethylsilyl triflate (TMSOTf), CH 2 Cl 2 , rt,; b) oxone, acetone, H 2 O, C 1 -C 6 Halogenated hydrocarbon, rt; c) AlI 3 , CH 3 CN, -40-80°C; d) MeONa, MeOH, CH 2 Cl 2 , -78-40°C; e) NaBH 4 , i-PrOH, CH 2 Cl 2 , rt; f) NaOH, CH 3 OH, H 2 O, 60°C
[0052] (1) Under glycosidation conditions, diosgenin reacts with 2,2,2-trimethylacetyl (Piv)-protected glucose trichloroimide ester donor I to obtain fully protected spirosteroid saponin II; (2 ) II is oxidized with oxone to obtain the product III of the double bond and 16-position oxidation; (3) III and AlI 3 The reaction obtains the product IV of epoxy removal while 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com