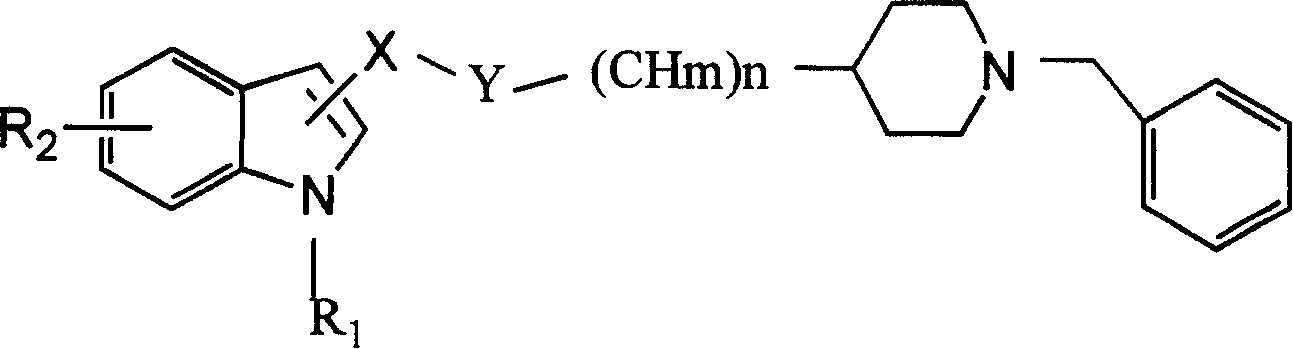Indolylpiperidine compound and preparation process and use thereof
A technology of indolylpiperidine and compounds, which is applied in the field of synthesis of indolylpiperidine derivatives, and can solve the problems of poor availability of donepezil biological agents and low toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: N-(indole-2-formyl)-(1-benzyl-4-piperidinyl)methanamine
[0037] Suspend 0.3g of 2-indolecarboxylic acid in 6ml of dry chloroform, cool in an ice-water bath, add 0.5ml of freshly treated thionyl chloride, let it warm up naturally, and stir overnight. The solvent was distilled off under reduced pressure (control temperature T<35°C). 10 ml of dry dichloromethane were added and evaporated to dryness. Repeat this treatment twice again to obtain a pale yellow solid, which was dissolved in 10ml of dichloromethane, cooled in an ice-water bath, and a solution of 270mg of 1-benzyl-4-piperidinemethylamine in 5ml of dichloromethane was added dropwise, and after the addition was complete, Add 0.3ml of triethylamine, stir at 0°C for 30min, wash the reaction solution twice with 20ml of water, and once with 15ml of saturated sodium carbonate solution, and dry the organic layer with anhydrous potassium carbonate. The solvent was evaporated, and the residue was passed throu...
Embodiment 2
[0039] Example 2: N-(indole-2-formyl)-2-(1-benzyl-4-piperidinyl)ethylamine
[0040] The procedure was as in Example 1, except that 1-benzyl-4-piperidinylethylamine was used instead of 1-benzyl-4-piperidinylmethylamine. Acicular needle crystals were obtained, MP=168~169°C
[0041] h 1 NMR (CDCl3, AM=400): 1.26~1.42(3H, m), 1.50~1.80(4H, m), 1.90~2.00(2H, m), 2.96(2H, m), 3.48~3.58(4H, m ), 6.12(1H,br), 6.70~7.66(10H,m), 9.52(1H,S)
Embodiment 3
[0042] Example 3: N-(1-methylindole-2-formyl)-1-(1-benzyl-4-piperidinyl)methanamine
[0043] The operation process is as in Example 1, except that indole-2-carboxylic acid is replaced with 1-methylindole-2-carboxylic acid. Needle crystals were obtained, MP=141~2°C
[0044] h 1 NMR (CDCl3, AM=400): 1.30~1.42 (2H, q-d), 1.60 (1H, m), 1.68~1.76 (2H, d), 1.90~2.00 (2H, t-d), 2.90 (2H, d), 3.32(2H, t), 3.50(2H, s), 4.02(3H, s), 6.22(1H, br), 6.78(1H, s), 7.08~7.60(9H, m)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com